 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 15(2); 2013 > Article |
|
Abstract
In spite of the best medical treatment, large hemispheric infarction, resulting from acute occlusion of either the internal carotid or the proximal middle cerebral artery with insufficient collateral blood flow is associated with a high case fatality rate of approximately 60%. Thus, a decompressive hemicraniectomy is considered a life-saving procedure for this devastating disease. Findings of three recent randomized, controlled clinical trials and their meta-analysis showed that early surgical decompression not only reduced the number of case fatalities but also increased the incidence of favorable outcomes. The authors review the pathophysiology, historical background in previous studies, operative timing, surgical technique and clinical outcomes of surgical decompression for malignant hemispheric infarction.
When patients present with acute occlusion of either the internal carotid artery (ICA) or the proximal middle cerebral artery (MCA) within the therapeutic time window for restoration of cerebral blood flow, rapid recanalization using intravenous/intra-arterial (IA) thrombolysis and IA mechanical embolectomy is the goal of acute stroke management.11) However, if the patient presents beyond the therapeutic time window, life-threatening brain swelling and herniation, known as malignant MCA infarction, can manifest within one week after the onset of stroke symptoms, along with a further decrease of consciousness and pupillary dilatation, necessitating early application of a decompressive hemicraniectomy for achievement of a better clinical outcome.24)
The present article reviews the pathophysiology, historical background in previous studies, operative timing, surgical technique and clinical outcomes of decompressive hemicraniectomy for malignant MCA infarction involving the MCA territory with or without anterior cerebral artery and posterior cerebral artery territories.
Acute occlusion of either the ICA or the proximal MCA and insufficient collateral blood flow result in cerebral infarction of the MCA territory and associated severe brain edema. Cytotoxic edema results from failure of sodium-potassium adenosine triphosphatase in brain cell membranes and is followed by vasogenic edema in association with disruption of the blood-brain barrier.
The space-occupying lesion inside the cranial vault increases the intracranial pressure, thereby reducing the cerebral perfusion pressure and cerebral blood flow. In particular, a space-occupying lesion in a unilateral hemisphere causes pressure gradients between the supratentorial and infratentorial compartments and between the bilateral supratentorial compartments. These pressure gradients then lead to transtentorial and subfalcine herniations. The transtentorial uncal herniation and the resultant displacement of the subthalamic-upper brainstem structures will impair the consciousness of the patient.
Thus, the aim of decompressive surgery is external herniation of the swollen infarcted brain for relief of brainstem compression and to reduce intracranial pressure.
The original purpose of decompressive hemicraniectomy was to help patients survive in cases of acute large hemispheric infarction.25) Various case reports, retrospective studies and trials have suggested that this surgical treatment lowers mortality without increasing the incidence of severely disabled survivors.8)13)15)23)27)29) Three European randomized, controlled clinical trials were conducted between 2001 and 2007.10)12)32) However, the DECIMAL (DEcompressive Craniectomy In MALignant middle cerebral artery infarction) trial in France and DESTINY (DEcompressive Surgery for the Treatment of malignant INfarction of the middle cerebral arterY) trial in Germany were stopped due to slow recruitment of cases and significant difference in mortality between groups.12)32)
Notwithstanding, the HAMLET (Hemicraniectomy After Middle cerebral artery infarction with Life-threatening Edema Trial) study, conducted in the Netherlands, was completed and published in 2009.10) Thirty two patients were randomly assigned to undergo surgical decompression and another 32 patients received the best medical treatment over a five-year period. According to the results, the use of hemicraniectomy resulted in a reduction in the number of case fatalities and poor outcomes (modified Rankin Scale score 5) for patients with large hemispheric infarction who were treated within 48 hours of stroke onset.
The clinical outcome for patients is considered to show improvement with early surgical decompression before or immediately after any neurological deterioration related to brain swelling.10)22)26)28)31)33)36) This clinical deterioration includes pupil asymmetry, an altered consciousness level and aggravated hemiplegia.6)
Patients commonly manifest such neurological deterioration within one week after the onset of stroke symptoms. In particular, one third of patients show deterioration within 24 hours, while another third show deterioration 24 - 48 hours after symptom onset.24)
To date, many clinical and radiological data defining early predictors of malignant hemispheric infarction have been reported.6)7)9)14)16)18)20)30)34) Malignant edema after acute infarction can be predicted based on the volume of the infarcted brain tissue, however, extension of the initial infarct territory, delayed spontaneous recanalization of the occluded vessel, hemorrhagic transformation of the infarcted brain and the fluid volume state of the patient can all make the prediction difficult. Thus, the predictors lack a sufficient predictive value with regard to selection of candidates for a decompressive hemicraniectomy prior to neurological deterioration. In addition, in order to ensure timely decompressive surgery, all patients with acute large hemispheric infarction should be observed in an intensive care unit or stroke unit setting. Thus, determination of strict cutoff criteria with a high specificity and positive predictive value for malignant infarction is needed.21)
The predictive value of the infarct volume assessed by early CT scan after stroke onset has not been found to be satisfactory, as follows: (1) hypodensity covering > 50% of MCA territory within five hours after symptom onset was predictive of a malignant course with a sensitivity of 61% and specificity of 94%;34) (2) hypodensity covering > 50% of MCA territory within 12 hours was predictive with a sensitivity of 64% and specificity of 66%;17) (3) hypodensity covering > 50% and 67% of MCA territory within 18 hours was predictive with a sensitivity of 58% and 45%, respectively and specificity of 94% and 100%, respectively.7)
One useful predictor is the initial infarct volume assessed using diffusion-weighted magnetic resonance (MR) imaging > 145 cm3 within 14 hours after acute MCA occlusion, which achieved a sensitivity of 100% and a specificity of 94% in the study by Oppenheim et al.20)
The cutoff criteria for lesion volume and associated midline brain shift for prediction of malignant edema should differ according to the timing after stroke onset.4)21) Gerriets et al. reported a midline shift ≥ 2.5, 3.5, 4.0 and 5.0 mm in transcranial color-coded duplex sonography as a predictor of malignant edema after 16, 24, 32 and 40 hours, respectively, after stroke onset with a specificity of 100% and positive predictive value of 100%.4)
Meanwhile, assessment of the final infarct volume can be performed using perfusion CT or perfusion MR images on admission, however, such perfusion parameters are still not optimal.3)30) In the MR perfusion study reported by Thomalla et al., perfusion lesion volume > 162 mL on a time-to-peak (TTP) map with a TTP delay threshold of > 4 seconds was found to predict malignant infarction with a sensitivity of 83% and specificity of 75%.30)
Conceptually, decompressive craniectomy procedures include both internal and external decompression. In the case of external decompression, the frontal, temporal and parietal bones overlying the infarcted hemisphere are removed, allowing for external herniation of the swollen infarcted brain.
With the patient in a state of general anesthesia, a skin incision is started just above the zygomatic arch 0.5 cm anterior to the tragus and then carried superiorly and posteriorly over the ear and around the parietal bone to the contralateral frontal midpupillary line. The hemicraniectomy then involves the removal of a large fronto-temporo-parietal bone flap, as large as possible. The craniectomy is limited by the following boundaries. The bone flap is made anteriorly in order to avoid violation of the frontal sinus, except in the case of a huge frontal sinus. The medial limit is 2 cm from the midline, in order to minimize venous bleeding on the dura. The posterior limit of the bone flap is approximately 5 - 6 cm posterior to the external auditory canal, thereby covering the MCA territory posteriorly and allowing for a neutral head position without compressing the brain. Inferiorly, the temporal squama is removed to the level of the zygomatic arch.
Following a stellate-shaped dural incision, it is commonly recommended that the infarcted brain tissue not be removed due to the presence of a salvageable penumbra area or viable tissue.10)12)32) However, the internal decompression (removal of the infracted brain tissue and/or an anterior temporal lobectomy) can be performed for patients with whole hemispheric infarction.19) Expansive duraplasty is then performed using a large flap of pericranial tissue or an artificial dura substitute. The dimensions of the expansive duraplasty should be extended in order to accommodate the subsequent aggravation of the brain edema.
Meticulous hemostasis is critical for prevention of a postoperative epidural hematoma. Multiple dural tenting sutures, bipolar coagulation of bleeding points on the dural surface, application of commercial hemostatic materials and placement of a closed suction drain in the epidural/subgaleal space are all used.
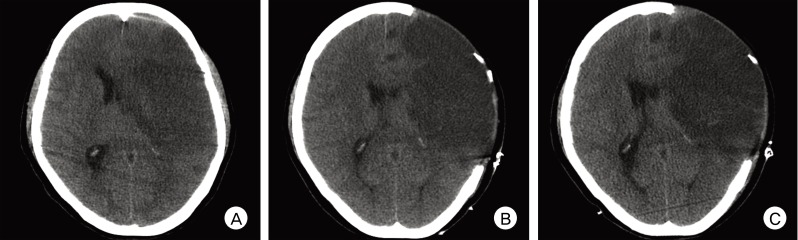
Finally, the temporalis muscle and skin flap are reapproximated and sutured layer by layer. However, the temporalis muscle and fascia can be resected in order to maximize external herniation of the swollen brain. Removal of the temporalis muscle does not cause problems with chewing, as the grinding phase of the closure stroke only requires one-third of the maximal bite force and only leads to a minimal decrease in the maximal bite force.1)5) Resection of the temporalis muscle and fascia provides a two-fold volume expansion on average when compared with the conventional technique on postoperative day 3 (Fig. 1).22)
Technical obstacles to obtaining the best external decompression are an insufficient craniectomy size, epidural/subgaleal hematoma, thick and swollen temporalis muscle, tough and inelastic temporalis fascia and tight scalp.2)22)35) Making hemicraniectomy as large as possible, meticulous hemostasis and resection of the temporalis muscle and fascia will all maximize external herniation of the infarcted brain.
The bone flap is stored in a tissue bank (< 70℃). Cranioplasty is then performed using the autogenous bone flap two to three months after the craniectomy.
After decompressive surgery for malignant MCA infarction, clinical outcomes at 6-12 months after stroke onset have been reported. For the criteria determining favorable and unfavorable outcomes, a dichotomization of the mRS score between 0-3 and 4 to death or between 0-4 and 5 to death has been used; however, an mRS score of 0-3 is most appropriate for a favorable outcome as it includes independent functional outcomes.
In the HAMLET study, the patients were randomly assigned to undergo surgical decompression or to receive the best medical treatment within four days of stroke onset.10) The decompressive-surgery group had a lower incidence of mortality (22% versus 59%) and mRS 5-6 (41% versus 59%) than the group that received the best medical treatment. However, the incidence of patients with mRS of 0-3 did not differ between the two groups (25% versus 25%).
Results of a pooled analysis of the three European randomized controlled trials indicated a better clinical outcome than that of the HAMLET study.31) Performance of decompressive surgery within 48 hours of stroke onset resulted in reduced mortality and an increase in the number of patients who had a favorable functional outcome. In addition, more patients in the decompressive-surgery group had mRS 0-3 (43% versus 21%) and mRS 0-4 (75% versus 24%) compared with those in the control group who received the best medical treatment.
In analysis of the three predefined subgroups of the DECIMAL trial, in the decompressive-surgery group, younger age showed correlation with a favorable outcome and there was a trend toward a worse outcome in patients with higher infarct volumes.32) However, no significant difference in the clinical outcome with mRS scores was observed between surviving patients with dominant and non-dominant hemisphere infarction.
In patients with malignant hemispheric infarction, decompressive surgery can reduce the number of cases of fatality and increase the number of favorable outcomes. In particular, if a decompressive hemicraniectomy is performed early, before irreversible cerebral herniation, using appropriate surgical techniques, favorable outcomes with functional independence can be achieved in a high proportion of patients.
NOTES
References
2. Andre C, Py Mde O, Niemeyer-Filho P. Temporal muscle haematoma as a cause of suboptimal haemicraniectomy; case report. Arq Neuropsiquiatr. 2003 9;61(3A):682-686;



3. Dittrich R, Kloska SP, Fischer T, Nam E, Ritter MA, Seidensticker P, et al. Accuracy of perfusion-CT in predicting malignant middle cerebral artery brain infarction. J Neurol. 2008 6;255(6):896-902;



4. Gerriets T, Stolz E, Konig S, Babacan S, Fiss I, Jauss M, et al. Sonographic monitoring of midline shift in space-occupying stroke: an early outcome predictor. Stroke. 2001 2;32(2):442-447;


5. Gibbs CH, Mahan PE, Lundeen HC, Brehnan K, Walsh EK, Sinkewiz SL, et al. Occlusal forces during chewing: influences of biting strength and food consistency. J Prosthet Dent. 1981 11;46(5):561-567;


6. Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996 4;53(4):309-315;


7. Haring HP, Dilitz E, Pallua A, Hessenberger G, Kampfl A, Pfausler B, et al. Attenuated corticomedullary contrast: an early cerebral computed tomography sign indicating malignant middle cerebral artery infarction: a case-control study. Stroke. 1999 5;30(5):1076-1082;


8. Harscher S, Reichart R, Terborg C, Hagemann G, Kalff R, Witte OW. Outcome after decompressive craniectomy in patients with severe ischemic stroke. Acta Neurochir (Wien). 2006 1;148(1):31-37; discussion 37.



9. Hofmeijer J, Algra A, Kappelle LJ, van der Worp HB. Predictors of life-threatening brain edema in middle cerebral artery infarction. Cerebrovasc Dis. 2008 2;25(1-2):176-184;


10. Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB. HAMLET investigators. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery Infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomized trial. Lancet Neurol. 2009 4;8(4):326-333;


11. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. American Heart Association Stroke Council. Council on Cardiovascular Nursing. Council on Peripheral Vascular Disease. Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013 3;44(3):870-947;


12. Juttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke. 2007 9;38(9):2518-2525;


13. Kalia KK, Yonas H. An aggressive approach to massive middle cerebral artery infarction. Arch Neurol. 1993 12;50(12):1293-1297;


14. Kasner SE, Demchuk AM, Berrouschot J, Schmutzhard E, Harms L, Verro P, et al. Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke. 2001 9;32(9):2117-2123;


15. Kilincer C, Asil T, Utku U, Hamamcioglu MK, Turgut N, Hicdonmez T, et al. Factors affecting the outcome of decompressive craniectomy for large hemispheric infarctions: a prospective cohort study. Acta Neurochir (Wien). 2005 6;147(6):587-594; discussion 594.



16. Krieger DW, Demchuk AM, Kasner SE, Jauss M, Hantson L. Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke. 1999 2;30(2):287-292;


17. Manno EM, Nichols DA, Fulgham JR, Wijdicks EF. Computed tomographic determinants of neurologic deterioration in patients with large middle cerebral artery infarctions. Mayo Clin Proc. 2003 2;78(2):156-160;


18. Mori K, Aoki A, Yamamoto T, Horinaka N, Maeda M. Aggressive decompressive surgery in patients with massive hemispheric embolic cerebral infarction associated with severe brain swelling. Acta Neurochir (Wien). 2001 143(5):483-491; discussion 491-2.



19. Nussbaum ES, Wolf AL, Sebring L, Mirvis S. Complete temporal lobectomy for surgical resuscitation of patients with transtentorial herniation secondary to unilateral hemispheric swelling. Neurosurgery. 1991 7;29(1):62-66;



20. Oppenheim C, Samson Y, Manai R, Lalam T, Vandamme X, Crozier S, et al. Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke. 2000 9;31(9):2175-2181;


21. Park J, Goh DH, Sung JK, Hwang YH, Kang DH, Kim Y. Timely assessment of infarct volume and brain atrophy in acute hemispheric infarction for early surgical decompression: strict cutoff criteria with high specificity. Acta Neurochir (Wien). 2012 1;154(1):79-85;



22. Park J, Kim E, Kim GJ, Hur YK, Guthikonda M. External decompressive craniectomy including resection of temporal muscle and fascia in malignant hemispheric infarction. J Neurosurg. 2009 1;110(1):101-105;


23. Pillai A, Menon SK, Kumar S, Rajeev K, Kumar A, Panikar D. Decompressive hemicraniectomy in malignant middle cerebral artery infarction: an analysis of long-term outcome and factors in patient selection. J Neurosurg. 2007 1;106(1):59-65;


24. Qureshi AI, Suarez JI, Yahia AM, Mohammad Y, Uzun G, Suri MF, et al. Timing of neurologic deterioration in massive middle cerebral artery infarction: a multicenter review. Crit Care Med. 2003 1;31(1):272-277;


25. Rengachary SS, Batnitzky S, Morantz RA, Arjunan K, Jeffries B. Hemicraniectomy for acute massive cerebral infarction. Neurosurgery. 1981 3;8(3):321-328;


26. Rieke K, Schwab S, Krieger D, von Kummer R, Aschoff A, Schuchardt V, et al. Decompressive surgery in space-occupying hemispheric infarction: results of an open, prospective trial. Crit Care Med. 1995 9;23(9):1576-1587;


27. Robertson SC, Lennarson P, Hasan DM, Traynelis VC. Clinical course and surgical management of massive cerebral infarction. Neurosurgery. 2004 7;55(1):55-61; discussion 61-2.



28. Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998 9;29(9):1888-1893;


29. Stolz E, Gerriets T, Babacan SS, Jauss M, Kraus J, Kaps M. Intracranial venous hemodynamics in patients with midline dislocation due to postischemic brain edema. Stroke. 2002 2;33(2):479-485;


30. Thomalla GJ, Kucinski T, Schoder V, Fiehler J, Knab R, Zeumer H, et al. Prediction of malignant middle cerebral artery infarction by early perfusion- and diffusion-weighted magnetic resonance imaging. Stroke. 2003 8;34(8):1892-1899;


31. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. DECIMAL, DESTINY and HAMLET investigators. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007 3;6(3):215-222;


32. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. DECIMAL Investigators. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 2007 9;38(9):2506-2517;


33. Vibbert M, Mayer SA. Early decompressive hemicraniectomy following malignant ischemic stroke: the crucial role of timing. Curr Neurol Neurosci Rep. 2010 1;10(1):1-3;



34. von Kummer R, Meyding-Lamade U, Forsting M, Rosin L, Rieke K, Hacke W, et al. Sensitivity and Prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol. 1994 1;15(1):9-15;


Fig. 1
Computed tomography (CT) scans taken before and after decompressive surgery, including a hemicraniectomy with resection of the temporalis muscle and fascia for acute middle cerebral artery (MCA) infarction. (A) Preoperative CT scan taken 20 hours after stroke onset shows an MCA territory infarction with a significant midline brain shift. (B) On postoperative CT scan one day after decompressive surgery, the craniectomy covers the whole MCA territory without any epidural/subgaleal hematoma and completely relieves the midline brain shift. (C) On postoperative CT scan three days after decompressive surgery, the overlying scalp successfully accommodates the aggravated brain swelling.
- TOOLS
-
METRICS

-
- 7 Crossref
- 0 Scopus
- 2,681 View
- 24 Download
- Related articles
-
Decompressive Craniectomy for Malignant Middle Cerebral Infarction.2009 June;11(2)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



