Superior ophthalmic approach in carotid-cavernous fistula: current concepts in indications, surgical techniques, and case reviews
Article information
Abstract
Carotid-cavernous fistulas, characterized by abnormal arteriovenous communication within the cavernous sinus (CS), can be classified as direct or indirect. Direct fistulas are defined as a direct connection between the internal carotid artery (ICA) and CS, whereas indirect fistulas result from an abnormal connection between the CS and dural arterial branches. The first-line treatment for both types of fistulas is endovascular intervention, most commonly accomplished through the transarterial and transvenous approaches of the conventional pathway, including the ICA, inferior and superior petrosal sinuses, or basilar plexus. Nonetheless, a retrograde approach through the superior ophthalmic vein may be necessary for individuals in whom conventional endovascular treatment fails. Herein, the current principles of surgical indication and technique are presented, along with case studies.
INTRODUCTION
Abnormal arteriovenous communication between the arteries and veins within the cavernous sinus (CS) results in a carotid-cavernous fistula (CCF). It is broadly classified into four types according to the Barrow classification: direct fistula (Barrow type A) and indirect fistulas (types B, C, and D) [4].
Direct fistulas are distinguished by a direct connection between the internal carotid artery (ICA) and CS. They are more prevalent in young males following head trauma and can be fatal [4]. The traumatic CCFs account for >50% of all direct CCFs [7]. Indirect CCFs are also known as cavernous sinus dural arteriovenous fistulas (CS-dAVF) when an abnormal communication occurs between the CS and dural arterial branches from the internal or external carotid artery or both. They are often low-flow fistulas as opposed the high-flow fistulas associated with direct CCF. However, CS-dAVF is common in postmenopausal women and patients with hypertension or atherosclerotic diseases [4,17].
Compromised blood flow within the CS and interference with normal venous drainage cause various symptoms of CCFs, including bruit, chemosis, proptosis, diplopia, blurred vision, headache, and even seizure [1,4,18,24]. The flow of fistula draining anteriorly is more likely to cause ocular symptoms of proptosis, chemosis, and even loss of vision [18]. Patients with flow to the cortical vessels or Sylvian’s vein may develop neurologic symptoms, such as intracranial hemorrhage, confusion, and seizures. Posteriorly draining fistulas commonly present with no symptoms, no bruit, or diplopia from isolated ocular motor nerve pareses, or “white-eyed” painful diplopia [26]. Symptoms and signs typically develop rapidly in direct CCF, but more indolently in dAVF. Intrinsically, the clinical symptoms do not always exhibit the aforementioned particular pattern but might exhibit a mixed pattern.
Regardless of the type of fistula, the majority of patients that require treatment can be managed with few complications owing to the robust development of endovascular interventional techniques [8]. The optimal therapeutic strategy is contingent upon the arterial supply, venous drainage, and patency of the circle of Willis. The transarterial route via the ICA is most frequently used in direct CCF [3,5,6]. Although a watchful waiting approach is reasonable in many patients with CS-dAVF, treatment is sometimes required to prevent long-term sequelae. Endovascular intervention is indicated for uncontrolled intraocular pressure, indefinable extraocular movement restriction, severe proptosis, choroidal detachment, and optic neuropathy. The inferior petrosal sinus (IPS) is the primary access point since it is the shortest and easiest path to the CS [13]. When the IPS approach is not possible due to anatomic variations, vascular malformation, deformation, or thrombosis, an alternative route should be considered. Moreover, an intrinsically intricate structure of the CS contains multiple neuro vasculature that sometimes hinders the approach of a catheter to the anterosuperior part of the CS where the opening of the Sylvian’s vein and superior ophthalmic vein (SOV) is located [16]. Embolization of the CCF via the SOV has been regarded as a successful alternative to the IPS or even the transarterial approach [15,19-21]. Fragile, undersized, thrombosed, or related vascular abnormalities may occasionally impede cannulation of the SOV; however, the effectiveness of the SOV approach has been described even in the presence of SOV thrombosis and in a variety of conditions [15,20,21,27]. In this review, the author discusses the embolization of CS-dAVF as well as the direct CCF through the SOV method, along with their respective experiences.
SURGICAL TECHNIQUES OF THE SOV APPROACH
In the angiography suite, the anterior approach to the SOV is typically used to provide a direct route for retrograde catheter placement. The femoral artery is punctured in the standard manner before the anterior approach through the SOV for road mapping during catheterization. Initially, the position of the SOV is identified using Doppler ultrasound, and a silk suture is placed to the tarsal plate for traction. An incision line of approximately 1–1.5 cm is placed using a No. 15 knife along the lower border of the eyebrows or an incision could be placed in the eyelid crease. Using Westcott scissors, the orbicularis oculi muscle and orbital septum are meticulously dissected. The orbital fat is then exposed and gently dissected using toothless forceps and a cotton swab. Consequently, the SOV is visible around 1.5–2 cm above the lacrimal caruncle, which is slightly deeper than the periorbital’s upper boundary. Then, mosquito forceps or a muscle hook is inserted into the lower region of the SOV, and the middle part of the 4-0 silk thread is held using forceps. When the muscle hook is placed, a 4-0 silk is threaded through the hole of the muscle hook. The mosquito forceps or muscle hook are then pulled out from the area where they had entered. The middle part of the silk is cut, and the two thread strands are located in the distal and proximal parts of the SOV, respectively, and tied loosely on each side for easy manipulation and hemostasis in case of vascular rupture. After pulling the two strands, the SOV was maintained in tension. Once the SOV is secured, an Angiocath IV (BD Franklin Lakes, NJ, USA) catheter is used to puncture the vein within the middle third of the visible segment. Under live fluoroscopy, the micropuncture wire is introduced through the needle and guided into the CS. In general, the SOV runs medially to laterally toward the superior orbital fissure. Due to the likelihood of anatomical variances or vein loops, a lateral-to-medial approach may be considered if the wire does not pass easily in this path. The SOV sometimes forms a loop-like arch; therefore, surgeons need to carefully dissect the distal and proximal parts of the SOV to verify the direction of the CS. Using angiography through the femoral artery, the locations of the catheter, SOV, and CS were determined, and coil embolization was performed. Occasionally, it appears unattainable owing to arterial bleeding after the failure of the first touch; however, in most cases, it can be accomplished with suction and appropriate manipulation of the SOV using pre-positioned silk ties (Fig. 1).
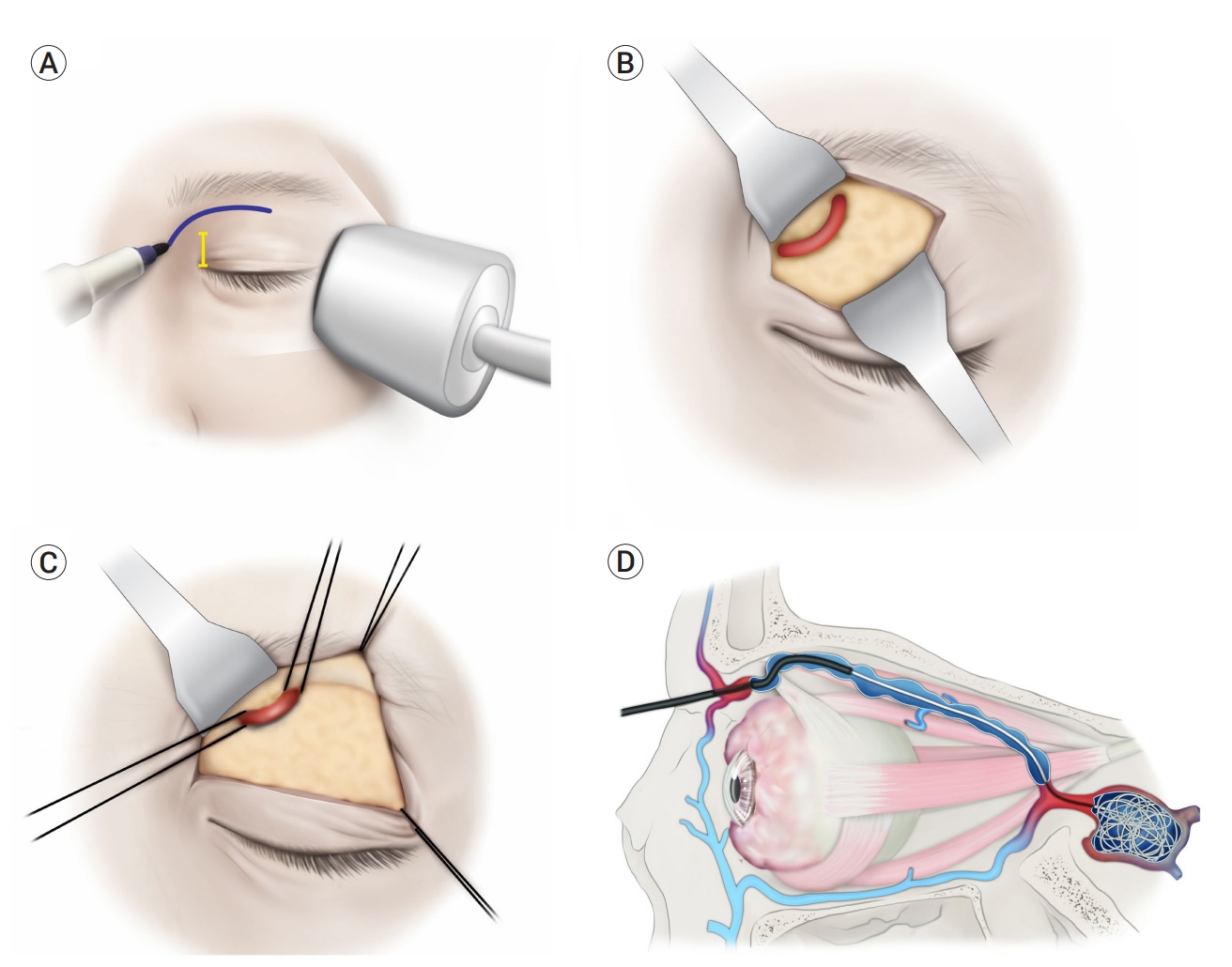
(A) First, the SOV is assessed using Doppler ultrasound, marking the incision line along the sub brow area. (B) The incision is placed on the skin, orbicularis oculi muscle, and septum to avoid unwanted damage to the SOV and surrounding tissues. (C) Traction is made using 4-0 silk on the distal and proximal sides of the superior ophthalmic vein to make it easy to handle and control any unexpected bleeding. (D) The microcatheter is inserted through the SOV, and the patient should show normal finally coiling embolization of the CS, especially around the entrance site of SOV [21]. SOV, superior ophthalmic vein; CS, cavernous sinus
SURGICAL INDICATIONS
IPS obstruction
The IPS originates from the posteroinferior aspect of the CS, and Shiu et al. classified the IPS junction into four types [23]. According to their definition, type I anatomy is characterized by a single IPS with a small diameter or no connection to the vertebral venous plexus (VVP). Type II is a single IPS with a large-diameter connection to the VVP, whereas Type III comprises a network of small veins that link to the internal jugular vein (IJV). In type IV anatomy, there is no connection between the IPS and IJV. A transvenous approach via the IPS is the usual route to the CS in the setting of CS-dAVF [8,14]. It is relatively direct and short from the IJV. Although some investigations have documented successful access to the CS via the occluded IPS, the fibrotic stricture between the end of the IPS and dilated CS makes final access to the CS difficult [11]. Thus, in the settings of fibrosis, thrombosis, and inadequate or absent IPS, the SOV approach is a credible alternative.
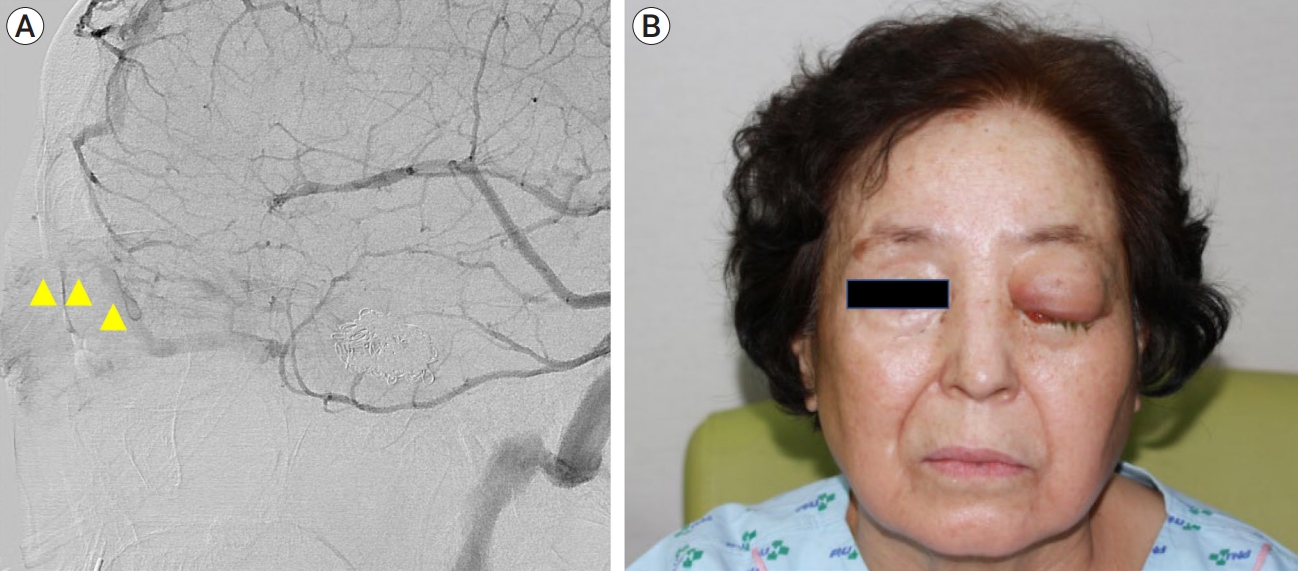
A 68-year-old woman with hypertension for 10 years sought treatment for a month history of right eye pain. The pain was pulsating, and the patient showed diplopia, abduction deficit in the right eye, and right eye injection. Angiography confirmed the presence of a CS-dAVF with IPS obstruction, and transvenous embolization attempts through the IPS were unsuccessful. Therefore, the SOV approach was attempted and vein cannulation was successful. Except for a mild incision line on the eyelid, there were no complications during the 6-month follow-up period (Fig. 2).

(A) Angiography reveals increased flow to the SOV with the IPS obstruction (yellow triangle) in a patient with CS-dAVF on the right side. (B) After the SOV approach, the flow to the SOV is well occluded. SOV, superior ophthalmic vein; IPS, inferior petrosal sinus; CS-dAVF, cavernous sinus dural arteriovenous fistulas
SOV thrombosis
In general, SOV thrombosis is associated with worsening orbital symptoms; however, in some instances, clots may spread posteriorly into the CS, resulting in spontaneous closure of the fistula [15,21,25]. Additionally, additional changes occur in the SOV after thrombosis, including a pinkish-pale coloring, vessel wall weakening and hardening. Therefore, major problems such as optic neuropathy, severe retinopathy, uncontrolled intraocular pressure, vision loss, cerebral venous drainage, and elevated intracranial pressure are relevant criteria for the SOV method [2]. When the vein is punctured, sometimes, little or no bleeding will occur, which makes it difficult for the surgeon to determine whether the SOV is punctured appropriately. However, once the thrombosed portion is passed, the catheter is filled with pulsating blood [21].
The majority of SOV attempts are successful; however, they may fail due to thrombosis of the SOV. According to previous studies, segmental thrombosis of the SOV hinders successful cannulation through the SOV [9,22]. Leibovitch et al. [15] also described an unsuccessful SOV approach, which was incapable of passing through the thrombosed anterior segment of the vein. They reported SOV cannulation in patients with extensive orbital dissection and multiple failed SOV approaches that were thrombosed.
In such cases, the plastic sheath of the angiocatheter can help push through the thrombosed SOV, and in our case (Fig. 3), we could traverse the soft obstruction caused by thrombosis. SOV thrombosis is often overlooked and causes unexpected failure of the SOV approach. On preoperative angiography, the surgeon should observe not only whether the CCF is still open, but also the continuity of blood flow and vessel integrity through the distal SOV.
Traumatic CCF
Direct and traumatic CCF are unlikely to close spontaneously, and when neurologic deficits or worsening venous congestion are present, endovascular embolization through the ICA is the most common treatment option. A guiding catheter is inserted into the ipsilateral femoral artery and advanced to the ICA, after which a microcatheter is inserted into the cavernous ICA, and subsequently through the fistula into the CS. Successful embolization of CCF can result in the immediate disappearance of symptoms, including proptosis, chemosis, and bruits. Ophthalmoplegia and optic neuropathy may require 4 and 6 months, respectively, to resolve [7,10].
Even with successful treatment, recanalization of the CCF or delayed exacerbation of orbital symptoms may occur, requiring transvenous treatment to occlude the opening of the SOV, as in our patient (Fig. 4). A 64-year-old woman presented with proptosis, increased intraocular pressure in the left eye, and seizures after falling. Computed tomography and angiography confirmed left direct CCF and obstructed IPS, IJV, and SOV enlargement. The symptoms of direct CCF were well recovered after combined transvenous-transarterial embolization 3 months after the first surgery; however, the CCF was recanalized. The high flow to the SOV was fully occluded through the retrograde SOV approach, which was used as an alternate pathway for the IPS, obstructing the conventional pathway.

Three months after the first coil embolization of traumatic CCF, the fistula is recanalized and the SOV is severely enlarged. (A) The IPS obstruction, which is a conventional pathway to block the flow to the SOV is observed. (B) Through the SOV approach, the opening of the SOV and Sylvian vein are successfully occluded, and no symptoms remain after surgery. CCF, carotid-cavernous fistula; SOV, superior ophthalmic vein; IPS, inferior petrosal sinus
Inaccessible to drainage opening
The CSs are a pair of 2 cm long and 1 cm wide structures located in the anterior region of the middle cranial fossa of the sphenoid bone. Multiple interconnected sinusoids support the venous architecture of the CS, allowing venous blood flow to be fairly sluggish and of low pressure. The ICA enters the carotid canal, passes through the petrosal canal, and then enters the medial aspect of the CS. The ICA is strongly attached to the CS by strong dural filaments, particularly at its entrance and exit by its inferior and superior ascending segments. The siphon portion traverses the CS, and the ICA may be observed traversing the CS on angiography (Fig. 5) [7,12]. This complex nature of the CS and surrounding structures allows up to 70% of CS-dAVFs to close spontaneously owing to local thrombosis; nonetheless, it hinders catheter movement from the posterior inferior region to the superior-anterior region of the CS. It is potentially problematic for transvenous coil embolization to obstruct blood flow to the SOV and Sylvian veins. A 51-year-old female patient, with an unsuccessful previous attempt to occlude the CS-dAVF using IPS, was also effectively treated with the SOV method. Four months postoperatively, no complications were observed.
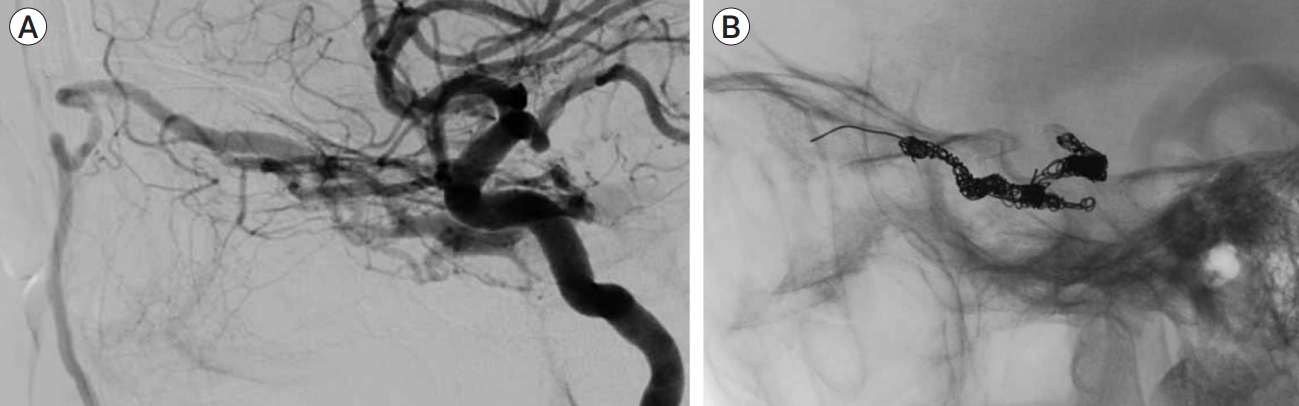
(A) Thrombosis and comparted nature of CS make it challenging to approach the opening of the SOV and sphenopalatine sinus even after overcoming the IPS obstruction. (B) Using the SOV approach, the superior-anterior part of the CS is fully obstructed, and no flow is observed. CS, cavernous sinus; SOV, superior ophthalmic vein; IPS, inferior petrosal sinus
POSTOPERATIVE MANAGEMENT
Patients should be informed preoperatively that embolization may exacerbate their signs and symptoms owing to acute thrombosis, inflammation, or the mass effect of the coil itself. This may include a new palsy; an increase in swelling and proptosis; and, in some scenarios, ischemia, or choroidal effusion. Most patients can be reassured that these symptoms will vanish within the next 4 to 8 weeks [15,20,21].
CONCLUSIONS
In conclusion, SOV embolization is an effective alternative treatment option for CS-dAVF as well as direct CCF if the conventional approach fails. With the help of a meticulous preoperative plan and intraoperative review of angiographic anatomy, SOV anatomical variations, fragility, and intravenous clotting should be determined before surgery. Additionally, patients should be informed of the complications and paradoxical postoperative worsening of symptoms, such as bleeding, vision loss, stroke, and even surgical failure. In any case, it is plausible to have neurosurgical and radio-interventional teams on hand in urgent situations.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.