Angiographic characteristics of ruptured versus unruptured vertebral artery dissecting aneurysm
Article information
Abstract
Objective
Vertebral artery dissecting aneurysm (VADA) is a rare and critical disease. VADA rupture can cause subarachnoid hemorrhage which is a major complication of VADA due to their high rebleeding rate and poor outcome. In the present study, ruptured and unruptured VADAs were compared by analyzing angiographic findings to determine useful predisposing factors for VADA rupture for appropriate treatment selection.
Methods
Subjects with VADA treated during a 10-year period were retrospectively identified. The 57 cases diagnosed with VADA were divided into ruptured (n=15) and unruptured (n=42) groups. In addition, each case was analyzed using angiographic 3-dimensional (3-D) reconstructed images. Factors such as length, dilated and stenotic diameter, shape, and vessel around the vertebral artery (VA) were measured and statistically compared.
Results
In the ruptured group, stenotic findings of the affected lesion were more common and severe than in the unruptured group. The average stenotic diameter was 2.27 mm (vs. 2.84 mm). And stenotic degree was 62% and 53% in the ruptured and unruptured groups, respectively. Posterior communicating artery (PcomA) flow was more common in the ruptured group (87% vs. 55%, p=0.028).
Conclusions
Based on angiographic findings, stenotic lesions, which may be influenced by PcomA flow, are more common in ruptured VADAs.
INTRODUCTION
Vertebral artery dissecting aneurysm (VADA) is a well-known rare disease which has been diagnosed more frequently because the diagnostic methods have improved over the last few decades. Endovascular treatments for VADA, such as trapping, coil embolization with or without stent, stenting, and using a flow diverter, have been investigated regarding their effectiveness and side effects. Conservative treatment is another option. Regarding the diverse methods, many factors should be considered for selecting the proper treatment when VADA is diagnosed [2,4,7-9,11,14,16].
The natural course of VADA shows a wide spectrum, from non-symptomatic to occlusive ischemic stroke and/or aneurysm rupture. Subarachnoid hemorrhage (SAH) is the major complication and shows poor prognosis due to high rebleeding and mortality rates in patients with VADA. Therefore, the evaluation of vessel wall condition is very important to determine the proper treatment strategy for VADA.
To decide the treatment strategy for unruptured intracranial aneurysms, such as the saccular type, the prediction of rupture risk using angiographic findings has been extensively researched [1,5,6]. Several parameters, such as the location, coexistence of daughter sac, and neck/height ratio, have been used to predict rupture risk of the unruptured aneurysm and could become therapeutic evidence for surgical and endovascular treatment. However, using the same parameters in dissecting aneurysms with different pathophysiological bases is difficult. In the present study, by comparing ruptured cases which are severe progressed type of VADA and unruptured cases of VADA, the significance of several parameters that could be used to evaluate VADA were validated.
MATERIALS AND METHODS
From April 2010 to July 2020, 57 patients diagnosed with VADA based on digital subtraction angiography (DSA) in one institute were included in the present study. Endovascular procedures were performed using an Allura Xper FD 20/20 system. The local Institutional Review Board of our medical center approved this retrospective study (IRB No. CR-21-064).
After dividing the subjects into a rupture group with SAH and unruptured group without SAH using CT or MRI images, each VADA group was analyzed using 2-dimensional (2-D) angiographic images and 3-D reconstructed images.
In addition, each case was classified based on dominancy of vertebral artery (VA) and relationship with posterior inferior cerebellar artery (PICA). Based on the angiographic image, each case was divided into the focal dilatation (small bulging without stenosis), fusiform dilatation (diffuse dilated portion without stenosis), and dilatation with stenosis (pearl and string) groups. The dilatation with stenosis group had both dilated and stenotic diameter than normal Vas [3,19–22]. Next, the lesion length (entire length of dissecting portion), most dilated diameter, most stenotic diameter of lesion segment as well as normal VA diameter and height (most dilated diameter–normal VA diameter), were measured for statistical comparison. When considering pathophysiology of VADA, degree of stenosis was calculated using the following formula: % of stenosis=[1−(most stenotic diameter/most dilated diameter)]×100 (Fig. 1).
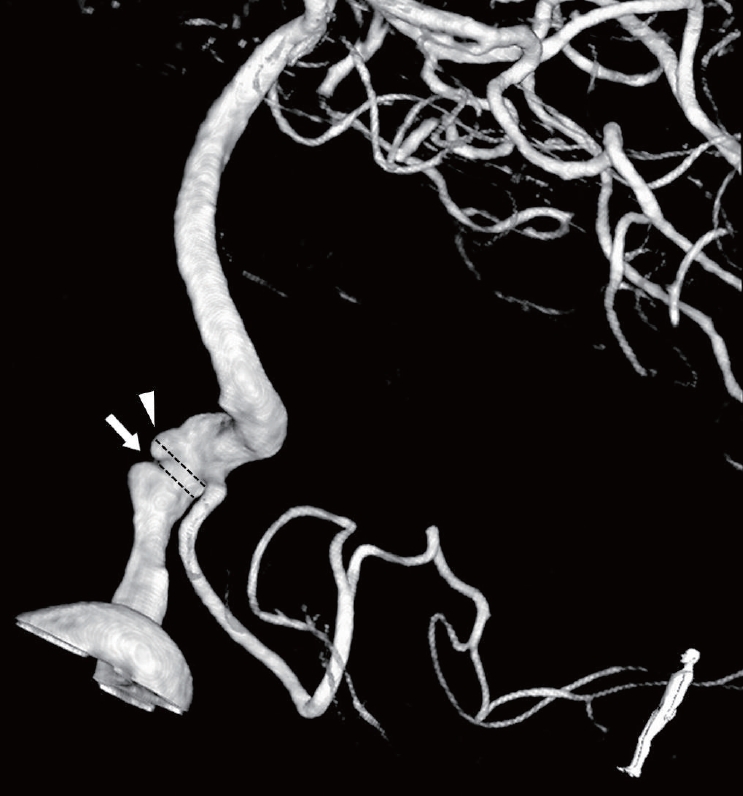
3-dimensional reconstructive angiography of 60-year-old man who has VADA with dilatation and stenotic lesion, Arrow: diameter of most stenotic portion, Arrow head: diameter of most dilated portion, VADA, vertebral artery dissecting aneurysm.
The relationship of the posterior communicating artery (PcomA) flow with the VADA rupture was analyzed by dividing the absence or presence of the PcomA based on the assumption that existence of the PcomA affects VA flow.
IBM SPSS statistics (IBM, NY, USA) was used for statistical calculations. The data normality was analyzed using the Shapiro-Wilk test. The independent t-test and chi-square test were used for comparisons between groups.
RESULTS
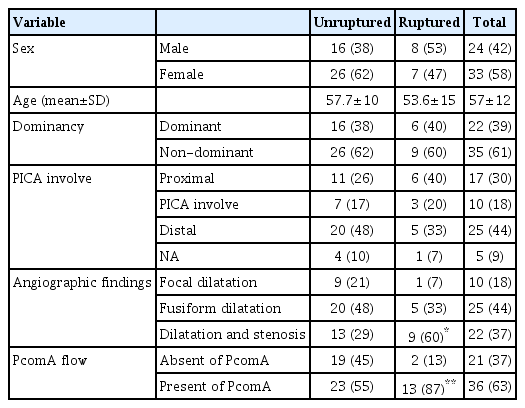
Among the 57 patients analyzed, 15 had ruptured aneurysms and 42 had unruptured aneurysms. The mean age was 57.7±10 years in unruptured cases and 53.6±15 years in ruptured cases. Patient demographic characteristics, including sex, age, dominancy of VA, and PICA involvement, were collected (Table 1).
Among the aneurysms, the dominant VADA was confirmed in 38.6% of the cases (40% ruptured, 38.1% unruptured). Aneurysms with PICA involvement were confirmed in 18% of the cases (20% ruptured, 17% unruptured). These factors were not statistically associated with rupture.
In angiographic findings, 37% of the cases (60% ruptured, 29% unruptured) had stenotic lesions, which was statistically more associated with rupture cases (p=0.03; Table 2). The mean length and dilated diameter of the affected segments were 9.34 mm and 6.32 mm in unruptured cases and 12.06 mm and 6.46 mm in ruptured cases, respectively, but without statistical significance.
In ruptured cases, the mean stenotic diameter was 2.27 mm, which was statistically significant (p=0.045) compared with unruptured cases (2.84 mm). In addition, degree of stenosis in the ruptured group was more severe than in the unruptured group (63% vs. 52%, p=0.048).
When analyzing with respect to the existence of PcomA, ruptured cases had significantly more PcomA involvement (87%) than unruptured cases (55%, p=0.028).
DISCUSSION
A gold-standard treatment for unruptured VADAs has not been established due to the variability of clinical cases. Numerous treatment methods, such as surgical approach, endovascular trapping, coil embolization with or without stent, stenting, and using a flow diverter, have been reported in many studies [4,7,10,11]. We have also treated VADA using various methods. Most of the ruptured VADAs were treated more aggressively; 9 of 15 cases were treated with trapping and 5 cases with coil embolization. Conversely, unruptured cases were treated more often with reconstructing methods, such as embolization, stenting or flow diversion, in our institute.
When deciding therapeutic plan for VADA, due to morbidity and mortality associated with VADA rupture and complications of treatments such as ischemic events, predicting the rupture is one of the most important aspects. Generally, aneurysm progression has been predicted using several angiographic findings such as size and morphological characteristics [1,5,6]. However, in the case of dissection, using the same parameters is not appropriate due to different pathophysiology. In the present study, useful parameters for estimating VADA were identified based on analysis of angiographic findings.
The arterial wall consists of three layers, the intima, media, and adventitia. Based on VADA pathophysiology, tearing of the arterial wall can cause intramural hematoma, intimal thickening, and ectasia that could lead to vessel stenosis and dilatation. Finally, tearing of the outermost layer of the intradural VA, the adventitia, causes SAH [2,16,17]. Due to this process, angiographic findings of dilatation and stenosis at the dissecting portion could indicate more progressed pathology at that portion. The findings vary among individuals. The degree of vessel dilatation and the length of affected segment do not differ significantly. However in cases of rupture, VADA, which has both dilatation and stenosis on angiographic findings, is more frequent. In addition, the diameter of the stenotic portion is narrower and degree of stenosis more severe than in unruptured cases, indicating that VADA, which shows more stenotic lesion on angiography, may have a more progressed vessel injury and higher possibility of rupture.
The blood stream velocity produces wall shear stress which is associated with aneurysm development. It is possible that the collateral flow, which could influence the velocity of the VA, is associated with the initiation and development of the dissection of the vessel. PcomA is the main collateral pathway connecting the anterior circulation and posterior circulation. Therefore, the PcomA flow could influence the entire posterior circulation including VA flow and development of VADA. In the present study, VADA rupture rate and the existence of PcomA were statistically correlated. PcomA flow tends to exist more in ruptured cases. Generally, high blood flow velocity can increase aneurysm development and rupture due to high wall shearing stress [15]. In a recent study, the maximum flow velocity of the basilar artery was increased with the presence of fetal PcomA based on phase contrast magnetic resonance angiography [23]. Potentially, the presence of PcomA is associated with the hemodynamic condition of VA and development of VADA.
Due to recent advances in endovascular treatment, it has been the method of choice in many VADA cases and internal trapping used as appropriate therapy. However, because of the risk of ischemic complications due to the occlusion of the vessel, reconstructive treatment, such as coil embolization with stents or flow diversion, is also acceptable [8,13,14,18]. The dominance of affected VA and the existence of PICA involvement are considered the major factors when selecting treatment due to their characteristic anatomy. Reportedly, PICA involvement is the significant risk factor for recurrence after endovascular treatment [9,12]. However, in the present study, these factors showed no significant difference statistically.
The present study had several limitations. First, the sample size was small and this was a single-center study. Second, selection bias may have existed because most cases were already treated for VADA. And further studies in which the hemodynamic effects of PcomA on the VA are investigated are needed.
CONCLUSIONS
Based on angiographic findings, stenotic lesions, which may be influenced by PcomA flow, are more common and severe in ruptured VADAs.
Therefore, careful monitoring strategies are needed to treat VADA coexisting with severe stenotic lesion and/or PcomA flow.
Notes
Disclosure
The authors have no conflicts of interest to declare concerning the materials or methods used in this study or the findings described.