Endovascular coil embolization for unruptured intracranial aneurysms in patients over 80 years of age
Article information
Abstract
Objective
As the average life span in modern society continues to increase, much interest is focused on high-risk procedures in elderly patients, including major surgical operations. We investigated the results of endovascular coiling of unruptured intracranial aneurysms (UIA) in patients over 80 years of age.
Methods
We retrospectively analyzed 39 patients aged over 80 years who underwent coil embolization for UIA between April 2007 and April 2019 at our hospital.
Results
Complete occlusion on digital subtraction angiography (DSA) immediately after surgery was performed in 44 (84.6%) of 52 cases of cerebral aneurysms. Four patients (7.7%) had residual aneurysmal necks, and four (7.7%) had contrast flow in the aneurysmal sac. Follow-up magnetic resonance angiography (mean: 8.2 months) was performed in 37 aneurysms in 24 patients. There was evidence of blood flow in the neck in seven cases (18.9%) and aneurysm in two cases (5.4%). Follow-up DSA (mean: 20.5 months) was performed in 14 aneurysms in 11 patients, and 11 aneurysms (78.6%) had complete occlusion, 1 aneurysm (7.1%) had an aneurysmal neck, and 2 aneurysms (14.3%) had contrast filling into the aneurysmal sac. Coil embolization procedure-related complications occurred in 3 patients (7.7%). Cerebral infarction occurred in 1 (2.6%), arterial dissection in 1 (2.6%), and hypoesthesia in 1 (2.6%).
Conclusions
Active treatment of UIA in elderly patients over 80 years of age through endovascular coil embolization can be considered.
INTRODUCTION
With improved health care services and increased average life span in modern society, the incidence of subarachnoid hemorrhage (SAH) and the discovery rate of unruptured intracranial aneurysm (UIA) in aged patients are increasing [5,6,9,12,13,16-18,22,26-28,33]. However, whether asymptomatic UIA should be treated actively in aged patients remains controversial.
Endovascular coil embolization of UIA has developed rapidly over the past decade and is used in the treatment of UIA [8,14,19,29,32]. Many researchers have revealed that selective embolization of cerebral aneurysm using coils shows lower morbidity and mortality rates than surgical cerebral aneurysmal neck clipping [2,3,15,20,25,30]. In other words, endovascular coil embolization can be used to treat various patients, including older patients, in whom surgical cerebral aneurysm clipping could be dangerous. However, there are few studies on the results of UIA coil embolization in elderly patients [1]. Thus, the present study aimed to investigate the results of endovascular coil embolization for UIA in elderly patients aged over 80 years in our single agency.
MATERIALS AND METHODS
Patient group
We performed endovascular coil embolization for 52 UIA in 39 patients aged over 80 years from April 2007 to April 2019. Nine men (23.1%) and 30 women (76.9%) with a mean age of 82.6 years (range: 80-91 years) were included. Patients who were over 80 years old at the time of surgery and treated for UIA via endovascular coil embolization as the first treatment were enrolled. Fusiform and traumatic intracranial aneurysms were excluded. Even if the patient had a small aneurysm, if the patient’s histories and aneurysmal characteristics were dangerous and determined its rupture risk was high, we performed coil embolization. These patient’s histories included a previous SAH and a family history of SAH. Dangerous aneurysmal characteristics included multilobed aneurysms, aneurysms with daughter sac, symptomatic aneurysms, and increasing aneurysm sizes. Patients’ medical histories, such as high blood pressure (30 individuals, 76.9%), diabetes (n=5, 12.8%), and cerebrovascular disease (n=6, 15.4%), were recorded. As symptoms at the time of hospitalization, headache, and dizziness, respectively, were the most prevalent at 9 individuals (23.1%), followed by 6 individuals (15.4%) who were discovered in a test for limb weakness, 4 (10.3%) who were discovered incidentally, 3 (7.7%) who were discovered in a test for dementia symptoms, 4 (10.3%) who had lid ptosis, and 1 (2.6%) had dysesthesia, facioplegia, memory loss, or stiffness of the neck, respectively (Table 1).
Characteristics of cerebral artery aneurysms
Among the 52 UIA cases, 4 (7.7%) had anterior cerebral artery (ACA) aneurysms, 7 (13.5%) had anterior communicating artery aneurysms, 14 (26.9%) had internal carotid artery (ICA) aneurysms, 14 had (26.9%) middle cerebral artery (MCA) aneurysms, 10 (19.2%) had posterior communicating artery aneurysms, and 3 (5.8%) had posterior circulation aneurysms. The number of small-sized cerebral aneurysms (<4 mm) was 12 (23.1%), while 31 were medium-sized (4-10 mm) (59.6%), and 9 (17.3%) were large-sized (>10 mm). The number of narrow-neck aneurysms (<4 mm) was 26 (50%) while 26 (50%) were wide (≥4 mm) (Table 2).
Embolization technique
All patients underwent digital subtraction angiography (DSA) for the bilateral carotid arteries and vertebral arteries before coil embolization. Coiling was performed under general anesthesia. The embolization strategy was planned using a biplane angiographic unit and a 3-dimension rotational angiogram. A guiding catheter with a guide wire was inserted into the internal carotid artery or vertebral artery. Using a road map with a working angle, a microcatheter was inserted carefully into the aneurysm along with the micro wire, and then the coils were inserted.
Among a total of 52 aneurysms, the single catheter technique was applied to 17 aneurysms (32.7%), the double catheter technique was applied to 16 (30.8%), and the stent-assisted technique applied to 19 (36.5%). In addition, 37 individuals (94.9%) were executed coil embolization via the femoral artery, but 2 (5.1%) were executed via the radial artery.
RESULTS
Angiographic results
Complete occlusion in DSA immediately after surgery was observed in 44 (84.6%) of 52 cerebral aneurysms. Four patients (7.7%) had residual aneurysmal necks, and 4 (7.7%) had contrast flow in the aneurysmal sac.
Follow-up magnetic resonance angiography (MRA) (mean: 8.2 months, range: 1-24 months) was performed in 37 aneurysms in 24 patients. There was evidence of blood flow in the neck in 7 cases (18.9%) and aneurysmal sac in 2 cases (5.4%) in the follow-up MRA. Follow-up DSA (mean: 20.5 months, range: 10-51 months) was performed in 14 aneurysms in 11 patients, and 11 aneurysms (78.6%) had complete occlusion, 1 aneurysm (7.1%) had aneurysmal neck, and 2 aneurysms (14.3%) had contrast filling into the aneurysmal sac. These two aneurysms that had contrast filling into the aneurysmal sac were treated with additional embolization (Table 3).
Clinical results
Coil embolization procedure-related complications occurred in 3 patients (7.7%). Cerebral infarction occurred in 1 (2.6%), arterial dissection in 1 (2.6%), and hypoesthesia in 1 (2.6%) (Table 4).
Cerebral infarction occurred in a patient with a left ICA aneurysm treated with stent-assisted coil embolization. Preoperative angiography revealed stenosis in the very proximal segment of the ICA before the aneurysm. Stent-assisted coil embolization was performed successfully without any problems. On postoperative day (POD) 8, right-sided weakness, dysarthria, and mental deterioration occurred. We performed DSA and found a thrombus around the stent. Therefore, we performed intra-arterial chemical thrombolysis, and the ICA was partially recanalized. Decreased mentality and weakness persisted in the patient (Fig. 1).

(A) Preoperative left internal carotid artery angiogram showing left internal carotid artery aneurysm and mild stenosis proximal to the aneurysm (arrow). (B) Postoperative left internal carotid artery angiogram showing left internal carotid artery aneurysm filled with coils (arrow). (C) Left internal carotid artery angiogram on post-embolization day 8 showing thrombus at the site of stent deployment (arrow) and occluded internal carotid artery by thrombus. (D) Left internal carotid artery angiogram after chemical thrombolysis showing a thrombus (arrow) and partially recanalized internal carotid artery.
Arterial dissection occurred in a patient who had a very tortuous left common carotid artery (CCA). During the insertion of the guiding catheter, arterial dissection occurred in the left CCA. After coil embolization, we performed stent insertion on the dissected segment. The patient was discharged from our hospital without thromboembolic complications (Fig. 2).
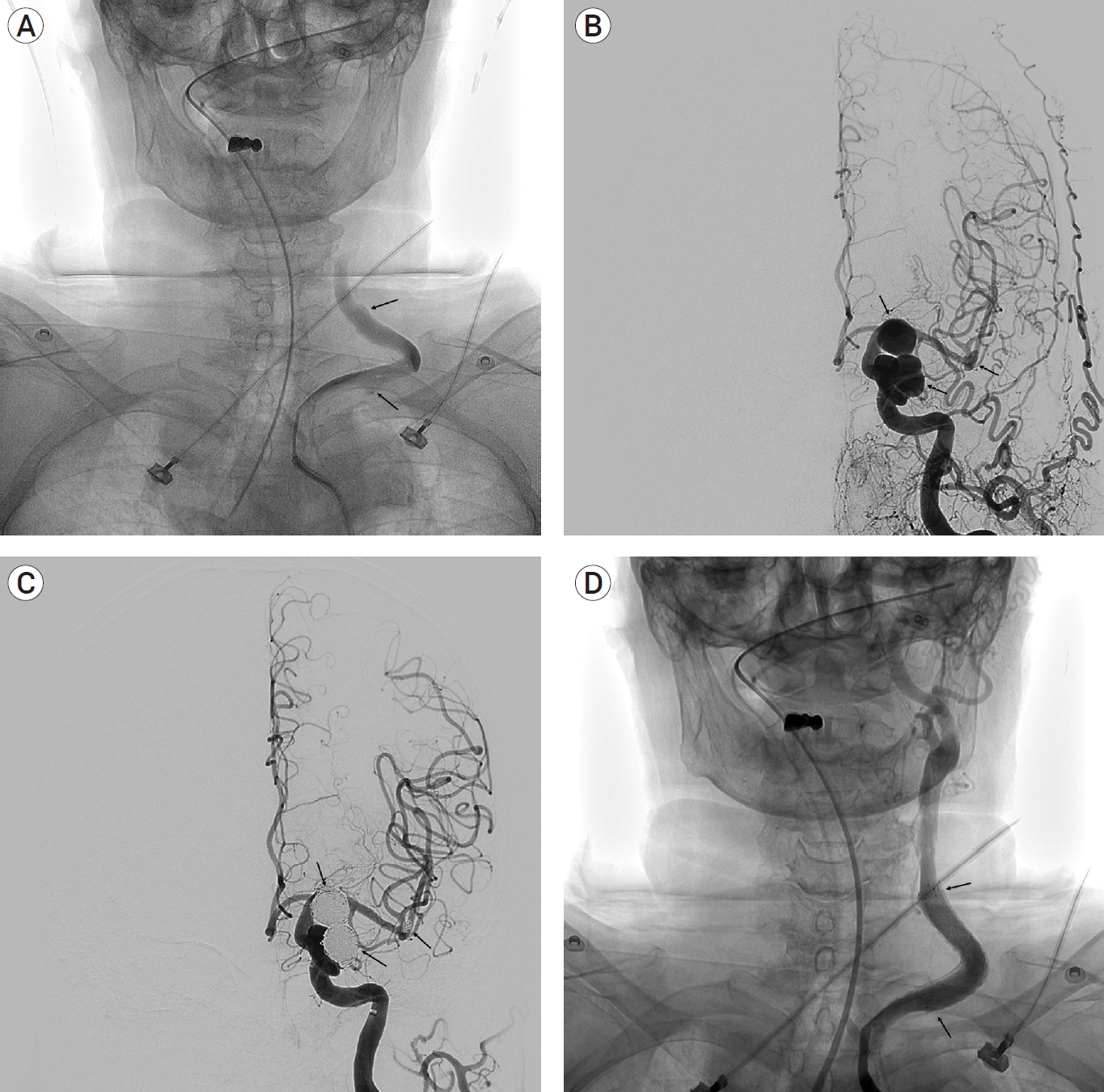
(A) The left common carotid artery angiogram showing severely tortuous proximal left common carotid artery with abnormal contrast stasis and vessel wall irregularities suggesting dissection (arrows). (B) Preoperative left common carotid artery angiogram showing left supraclinoid and paraclinoid internal carotid artery aneurysms and left middle cerebral artery bifurcation aneurysm (arrows). (C) Postoperative left internal carotid artery angiogram showing left supraclinoid and paraclinoid internal carotid artery aneurysms and left middle cerebral artery bifurcation aneurysm filled with coils (arrows). (D) Left common carotid artery angiogram after stent insertion in the dissected segment showing tolerable flow in the proximal left common carotid artery (arrows).
The hypoesthesia patient had a left MCA aneurysm and underwent coil embolization under stent assistance. On POD 3, hypoesthesia of the right hand occurred. In the diffusion-weighted image, there were diffuse microinfarctions in the left cerebral hemisphere, left basal ganglia, and right parietal lobe. Therefore, hydration and additional antiplatelet drugs were administered to the patient. Right hand hypoesthesia improved after conservative treatment.
DISCUSSION
There are numerous studies on the prevalence of cerebral aneurysms. One of them was a study about autopsies for patients who died in a hospital in Japan and the cerebral aneurysm was discovered in approximately 4.6% of the patients [13]. The important thing in this study was that the average age at which cerebral aneurysm rupture was 63.5 years and the average age at which UIA showed the highest prevalence rate was 80 years. Furthermore, according to the data released by Statistics Korea, the average life span of a Korean as of 2020 is 82.6 years and is gradually increasing. However, there are few studies on endovascular coil embolization in patients aged over 80 years; this prompted us to study elderly patients over 80 years of age.
The treatment of UIA in elderly patients has been controversial. Elderly patients have a lower life expectancy and generally more operative complications than young patients. Several studies on elderly patients who were treated for intracranial aneurysms reported procedure-related complications. Cai et al. and Hwang et al. reported 19% and 4.1% of procedure-related complications, respectively [4,11]. Gonzales et al. reported 6% procedure-related complications in patients who were treated for UIA [7]. In our results, complications related to endovascular coil embolization occurred in 3 patients (7.7%). The average age of the patients in our study was higher than that in other studies. This could lead to difficulty in the approach because of severely tortuous blood vessels, stenosis caused by atherosclerosis, and the possibility of arterial injury due to decreased arterial compliance and consequently, an increase in procedure-related complications. However, our study showed a similar complication rate compared to other studies.
In our cases, there were three procedure-related complications, including two thromboembolic complications and one arterial dissection. We share what we learned through these three cases with complications encountered while treating elderly patients with UIA. First, it should be considered that delayed thrombosis may occur if there is stenosis in the vessel segment when deploying the stent. Second, if the blood vessels are severely tortuous, the guiding catheter should be inserted more carefully. Third, cerebral infarction including areas unrelated to the procedure can occur; however, if there is no severe vessel occlusion, the patient can recover through conservative treatment.
Previous studies carried out on elderly patients who underwent endovascular coil embolization for UIA showed that aneurysmal size was large. Because elderly patients’ life expectancies and risks for small aneurysm rupture are low, surgeons do not tend to treat elderly patients with small aneurysms. Cai et al. and Gonzales et al. reported 30% and 37% of aneurysms below 12 mm and 10 mm in size in the elderly, respectively [4,7]. However, our study and Hwang et al reported 82.7% and 83.5% of aneurysms below 10 mm in size, respectively [11]. This means that we and Hwang et al actively treated elderly patients with small aneurysms [11]. Some studies reported that during coil embolization, small aneurysms have less thromboembolic complications and relapse rates than large aneurysms [24,30]. Our results (mortality: 0%, morbidity: 2.6%) and Hwang et al.’s results (mortality: 0%, morbidity: 0%) were good [11]. Therefore, we suggest that actively treating elderly patients with small aneurysms is worth considering.
In the present study, the insertion of devices including stents had a ratio of 36.5%. Cai et al. used the balloon-assisted technique (BAT) in 11% of patients and the stent-assisted technique (SAT) in 4% of patients [4]. Hwang et al used BATs in 9.8% and SAT in 8.2% [11]. Although the average aneurysm size in our study was small compared to other studies, there are several reasons for using more stents. First, compared to before, the incidence of complications gradually decreased as the experience of using stents and antiplatelet drugs increased, and as a result, the rate of coil embolization using stents increased overall. Second, it is difficult to use multiple catheters or balloons because of the relatively large number of intracranial stenosis in elderly Asians. Third, various improved stents have been released, making it easier to safely deploy stents to desired locations even for elderly people with many tortuous blood vessels. Because of these reasons, our study inserted more stent devices than other studies. It was expected that if the percentage of intravascular device insertion, including the stent, increases, more thromboembolic events would occur [10,21,23,31]. However, in our study, dual antiplatelet therapy was given during the week before surgery and was maintained for 1 year after surgery, after which the patient was switched to aspirin monotherapy. If necessary, intra-arterial infusion with an antiplatelet agent was administered during the procedure, and efforts to minimize the time required for the procedure were made. Therefore, the thromboembolic complication that left the patient with permanent neurological disability occurred in one patient (2.6%) without any hemorrhagic complications.
CONCLUSIONS
The aggressive treatment of UIA in elderly patients remains controversial. Despite the few cases in our study, based on our results, if patients’ histories or aneurysmal characteristics are likely to rupture, active treatment of UIA even in elderly patients over 80 years of age through endovascular coil embolization is worth considering.
Notes
Disclosure
The authors report no conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper.