Usefulness of sectional images in dural AVF for the interpretation of venous anatomy
Article information
Abstract
Knowledge of the venous anatomy is essential for appropriately treating dural arteriovenous fistulas (AVFs). It is challenging to determine the overall venous structure despite performing selective angiography for dural AVFs with feeder from multiple selected arteries. This is because only a part of the veins can be observed through the shunt in the selected artery. Therefore, after performing selective angiography of all vessels to understand the approximate venous anatomy, the venous anatomy can be easily understood by closely examining the source image of computed tomographic angiography or magnetic resonance angiography. Through this, it is possible to specify the vein that is to be blocked (target embolization), thereby avoiding extensive blocking of the vein and avoiding various complications. In the case of dural AVF with feeder from single selected artery, if the multiplanar reconstruction image of the three-dimensional rotational computed tomography obtained by performing angiography is analyzed thoroughly, a shunted pouch can be identified. If embolization is performed by targeting this area, unnecessary sinus total packing can be avoided.
INTRODUCTION
Diseases such as pial arteriovenous malformations (AVMs), pial arteriovenous fistulas (AVFs), dural AVF, and direct-type carotid-cavernous fistula are characterized by the brain and dural arterial and venous direct connections without passing through the capillary. In pial AVM and AVF, blood vessels formed with a nidus or shunt are therapeutic targets; therefore, interpretation of the venous anatomy may be less important for establishing a treatment strategy. The therapeutic target of dural AVF is the sinus wall where the fistula is formed. Currently, there is no way of blocking only the sinus wall using medical technology; therefore, it is necessary to block the sinus lumen adjacent to the sinus wall where the fistula is formed. Therefore, understanding the anatomy of the vein connected to the fistula is essential to determine the minimum extent of the sinus lumen to be blocked and predict whether normal venous drainage will be affected when it is blocked.
CEREBRAL ANGIOGRAPHY AND SECTIONAL IMAGES IN DURAL AVF
The gold standard for diagnosing a cerebrovascular disease is digital subtraction selective angiography, which directly selects an artery going into the brain and injects a contrast agent. The venous structure is very curved and overlapping, making it challenging to understand using only the anteroposterior (AP) and lateral view projection images of angiography. Currently, three-dimensional (3D) rotational computed tomography (CT) (cone beam CT) is available in most angiographic suites; therefore, multiplanar reconstruction (MPR) images obtained by performing 3D rotational CT allow for easier interpretation of the venous anatomy. Digital subtraction selective angiography is performed by selecting one artery. If the dural AVF is connected to the middle meningeal and occipital arteries, which are the branches of the right external carotid artery (ECA), a contrast agent can be filled in all fistulas and veins connected to these fistulas by selecting the right ECA. The venous anatomy connected to the dural AVF can be identified at once by observing the MPR images of 3D rotational CT performed in this manner. However, if the right and left ECA branches simultaneously supply blood to one dural AVF, it is challenging to identify the entire venous anatomy using only one selective angiogram. This is because only part of the vein appears through the fistula connected to the selected artery, and the contrast medium cannot fill the entire vein. If both femoral arteries are punctured, the left and right ECAs are selected as catheters, and angiography is performed while injecting the contrast medium simultaneously, and the entire venous anatomy can be obtained. However, this situation is rare. Then, after approximately identifying the venous anatomy through angiography and MPR imaging of 3D rotational CT performed after selecting the right and left ECAs, respectively, the venous anatomy can be easily understood by closely examining the CT angiography (CTA) or magnetic resonance angiography (MRA) source image. If we observe the CTA or MRA source image from the beginning, without observing the angiography and MPR image of the 3D rotational CT, both the artery and vein appear white and cannot be distinguished. However, after an approximate assumption of the venous anatomy through angiography and the MPR image of 3D rotational CT, observing the CTA or MRA source image allows us to distinguish between arteries and veins and understand the venous anatomy created by the assumed reconstruction. Performing the fusion technique is possible by using the latest angiography machine. It is possible to create an image by fusing each MPR image of the 3D rotational CT performed by selecting each blood vessel through this technique. Thus, the entire venous anatomy can be identified. However, this technique has limitations because it is only possible with the latest, expensive machines.
IMPORTANCE OF SECTIONAL IMAGES ANALYZING VENOUS ANATOMY IN DURAL AVF
The sectional image mentioned in the title is the CTA and MRA source image and the MPR image of the 3D rotational CT performed by the angiography machine. The AP and lateral projection images of digital subtraction angiography are important methods for diagnosing cerebrovascular diseases and establishing treatment plans. However, it is not sufficient to identify the venous anatomy in dural AVF. A sectional image is essential to identify such complex venous anatomy; therefore, 3D rotational CT should be performed after angiography to obtain an MPR image. By comparing and analyzing the MPR image, which is a sectional image obtained in this manner, with the MRA or CTA source image, which is the same sectional image, accurate identification of the venous anatomy is possible.
It is important to analyze the anatomy of the vein in a dural AVF for the following reasons. First, as mentioned above, for the fundamental treatment of a dural AVF, it is necessary to block the sinus lumen after passing through the fistula in the sinus wall. Analysis of the venous anatomy is essential to avoid extensive total sinus packing and determine the sinus lumen that needs blocking.
By analyzing the MPR image of the 3D rotational CT performed in a patient with dural AVF, it was observed that a tubular or elliptical vascular structure where the dural AVF was formed was in the venous pouch connected to the sinus but separated from the main sinus. This is referred to as a shunted pouch [3,l4]. Therefore, if only the shunted pouch is selected and blocked in such a patient, venous drainage through the sinus can be preverved, and total sinus packing can be avoided.
Second, venous structures that affect normal venous drainage can be avoided. If the vein is blocked with a material such as a coil without a clear sense of purpose, a vein that does not need to be blocked can be blocked. There may be inevitable cases for treatment, but even so, efforts should be made to avoid these venous structures. If such efforts are insufficient, normal venous drainage can be disturbed, and in the worst case, venous hypertension, infarction, and hemorrhage can occur.
TREATMENT OPTION OF DURAL AVF
In dural AVFs, the fistula forms in the sinus wall. Despite advances in medical technology, no technology can block only the fistula point formed in the sinus wall. In the past, when adequate liquid embolic material was not available, the venous approach was considered the appropriate treatment. This is because when using a particle embolic material such as Gelfoam in an arterial approach, only the artery is blocked, and the fistula in the sinus wall remains unblocked. Although angiography performed immediately after treatment revealed appropriate blocking, the recurrence of dural AVF observed on follow-up angiography was common because the disease source persisted. Therefore, blocking the sinus lumen using a coil with transvenous approach adjacent to the sinus wall with fistulas is considered a fundamental treatment. Currently, ONYX, a highly permeable liquid embolic material, is used. As this material is highly permeable, it is injected through an arterial approach by selecting an appropriate artery, passes through the selected artery, and blocks the multiple feeders and fistula in the sinus wall, reaching the sinus lumen and blocking it (Fig. 1). Even if ONYX is injected via a venous approach, ONYX fills the multiple feeders after passing the sinus lumen and blocks the fistula [1,2,6]. The fistula located on the sinus wall is blocked with ONYX; therefore, fundamental treatment is possible. Herein, we analyzed the venous anatomy in dural AVFs and explained its clinical application through a case review.
CASE 1
A 55-year-old female visited the hospital with right periorbital swelling for a week with numbness around the forehead for 5 days. Physical examination revealed periorbital swelling, pupil dilatation, medial and lateral gaze limitations, and forehead numbness with respect to the right side. Based on this, a diagnosis of cranial nerve palsy of III, VI, and V1 was established.
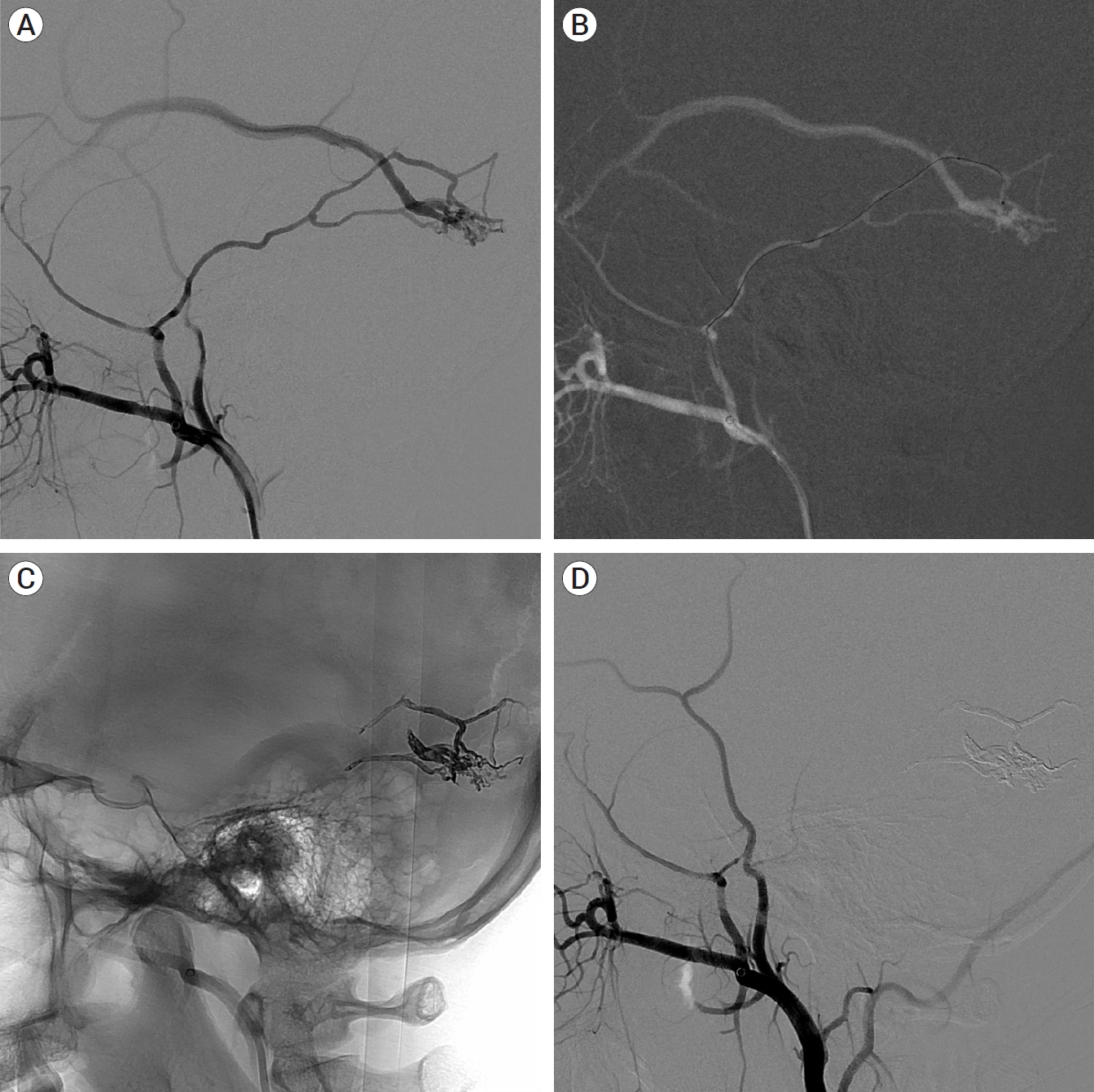
MRA and selective digital subtraction angiography were performed to determine the cause. In the angiography of the right internal carotid artery (ICA) selection, multiple dural branches in the posterior genu of the ICA, fistulas in the superior and posterior region of the right cavernous sinus, and contrast medium filled in the cavernous sinus were observed. In addition, a venous pouch without a fistula was observed in the inferior and posterolateral regions of the cavernous sinus, along with venous drainage into the inferior petrosal sinus in the inferior and posteromedial regions. At this point, only a part of the cavernous sinus can be observed. In the medial portion of the cavernous sinus, two intercavernous sinuses are visible, with the left cavernous sinus and the left inferior petrosal sinus connected to the two intercavernous sinuses (Fig. 2A). Angiography of the right ECA selection revealed that multiple feeding arteries from the right internal maxillary artery and ascending pharyngeal artery formed fistulas in the inferior and anterior portions of the right cavernous sinus, with dilation of the right superior ophthalmic vein. In addition, two intercavernous sinuses were observed medially (Fig. 2B). Angiography of the left ECA selection revealed multiple feeders originating from the left ascending pharyngeal artery and left internal maxillary artery connected to the venous pouch in the medial portion of the right cavernous sinus, which were not observed on the previous angiography, forming a shunted pouch. The right cavernous sinus was completely filled, and the right superior ophthalmic vein was dilated, with drainage through the right inferior petrosal sinus (Fig. 2C, D). Angiography of the left ICA revealed multiple dural branches originating from the posterior genu that were visible and connected to another shunted pouch (Fig. 2E). Angiography performed by selecting the above four blood vessels revealed veins with varying anatomies; therefore, it is necessary to comprehensively consider the partially visible venous anatomy. The venous anatomy can be accurately predicted by combining all these findings, drawing a schematic diagram of the venous anatomy (Fig. 2F), and comparing and analyzing it with source images of MRA (Fig. 3A-D).
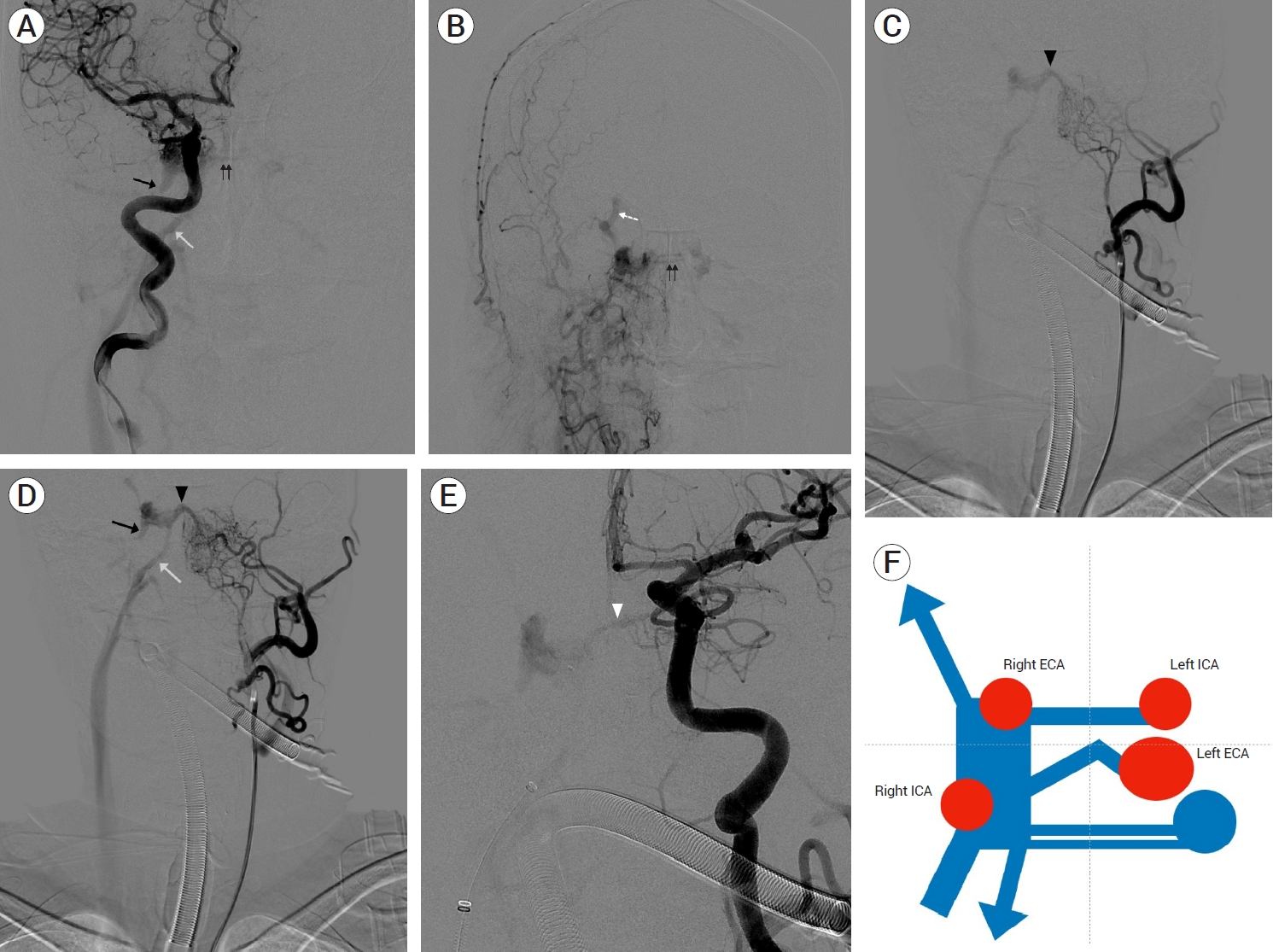
Case 1. Dural AVF in right cavernous sinus. (A) Right ICA selective angiography, AP view. (B) Right ECA selective angiography, AP view. (C, D) Left ECA selective angiography, AP view. (E) Left ICA selective angiography, AP view. Non shunted venous pouch (black arrow). Right inferior petrosal sinus (white arrow). Intercavernous sinus (black double arrows). Right superior ophthalmic vein (white dot arrow). Shunted pouch connecting to left ECA (black arrow head). Shunted pouch connecting to left ICA (white arrow head) in A, B, C, D, and E. (F) Illustration of venous anatomy in dural AVF. ICA, internal carotid artery; AP, anteroposterior; ECA, external carotid artery; AVF, arteriovenous fistula

Case 1. Source images of MRA in A, B, C, and D. (A) Non shunted pouch (white arrow). (B) Shunted pouch connecting to left ECA (white arrow). (C) Intercavernous sinus (white arrow). (D) Shunted pouch connecting to left ICA (white arrow). (E, F) Transvenous ONYX and coil embolization of dural AVF in cavernous sinus. MRA, magnetic resonance angiography; ECA, external carotid artery; ICA, internal carotid artery; AVF, arteriovenous fistula
Based on this, when establishing a treatment plan, the venous approach was preferred because the feeders were very thin and numerous. The shunted pouch shown in the left ECA selective angiography was treated by transvenous approach and selecting the shunted pouch, and multiple feeders originating from the right ECA and ICA were diffusely connected to the right cavernous sinus; therefore, it was treated by blocking the right cavernous sinus. In the shunted pouch connected to the left ICA, the embolic material might have flowed into the left ICA, causing an infarction; therefore, radiotherapy was planned. The shunted pouch connected to the multiple feeders of the left ECA was selected using a microcatheter, and ONYX was injected. After injection, it could be observed that not only the sinus lumen but also the multiple feeders were embolized (Fig. 3E). Thereafter, the dural AVF disappeared on the left ECA selective angiography. To treat the dural AVF of the cavernous sinus connected to the right ECA and ICA, the right cavernous sinus was selected using a microcatheter, and sinus total packing was performed using a coil (Fig. 3F). On angiography performed subsequently, the dural AVF was not observed. Novalis radiosurgery was performed for the shunted pouch connected to the multiple feeders in the untreated left ICA, and all symptoms were resolved after 2 months. Angiography performed after a year revealed the absence of the dural AVF in the right cavernous sinus. A known aneurysm of the left middle cerebral artery at the bifurcation level was subsequently coil-embolized. However, a new dural AVF was observed in the left transverse sinus, which was not observed in the previous examination (Fig. 4).

Case 1. 1 year follow up angiography. No recurrence of dural AVF in right cavernous sinus. (A) Right ICA selective angiography, AP view. (B) Right ECA selective angiography, lateral view. (C) Left ICA selective angiography, AP view. Known aneurysm in left MCA bifurcation area which was coil-embolized. (D) Left ECA selective angiography, lateral view. De novo development of dural AVF in left transverse sinus. AVF, arteriovenous fistula; ICA, internal carotid artery; AP, anteroposterior; ECA, external carotid artery
CASE 2
A 61-year-old male visited our hospital with a headache. Angiography of the left ECA revealed multiple feeding arteries from the left middle meningeal artery and left occipital artery forming a dural AVF around the left transverse sinus (Fig. 5A). In addition, angiography of the left vertebral artery (VA) revealed that the left occipital artery had formed a dural AVF in the same area (Fig. 5B). As reflux and ectasia were observed in the cortical vein, a Borden class 3 and Cognard class 4 lesion was diagnosed that warranted treatment (Fig. 5C). In the coronal section of the MPR images of the 3D rotational CT performed by selecting the left ECA, a venous pouch connected to the transverse sinus, with the fistula connected to this pouch was observed, indicating the presence of a shunted pouch (Fig. 5D-F). If so, the treatment should aim to preserve the transverse sinus to maintain normal venous drainage and block only the shunted pouch. The posterior branch of the middle meningeal artery was well developed; therefore, we decided to treat it through this artery.

Case 2. Dural AVF in left transeverse sinus. (A, B) Left ECA selective angiography, lateral view. (C) Left VA selective angiography, lateral view. (D, E, F) MPR images of 3D rotational CT. AVF, arteriovenous fistula; ECA, external carotid artery; VA, vertebral artery; MPR, multiplanar reconstruction; 3D, three-dimensional; CT, computed tomography
ONYX was injected after selecting the posterior branch of the left middle meningeal artery as a microcatheter. ONYX filled the shunted pouch, and the surrounding feeding arteries were filled (Fig. 6A-C). Subsequently, the feeding artery and dural AVF, observed on the previous angiography of the left ECA and left VA, were occluded on angiography, and normal venous flow through the left transverse sinus was preserved (Fig. 6D-F). To avoid the ONYX migration into the transverse sinus, transarterial ONYX embolization with ballooning in the transverse sinus might be considered [5].

Case 2. Transarteral ONYX embolization and follow up angiography. (A, B, C) Transarteral ONYX embolization of dural AVF in transeverse sinus. (D, E, F) After treatment, immediately follow up angiography. Total occlusion of dural AVF and preserved venous flow through left transeverse sinus. (G, H, I) 1 year follow up angiography. No recurrence of dural AVF and preserved venous flow through left transeverse sinus. AVF, arteriovenous fistula
Follow-up angiography after a year revealed that the dural AVF was completely resolved, and the left transverse sinus was also intact (Fig. 6G-I). Herein, unlike in the previous case, since the complete venous anatomy could be observed on the right ECA selective angiography, the venous anatomy could be identified by creating and analyzing an MPR images of the 3D rotational CT.
CASE 3
A 60-year-old male visited our hospital with aphasia. Angiography of the left ECA revealed a dural AVF with multiple feeding arteries from the left occipital artery in the left transverse sinus. Since the distal area of the left transverse sinus was occluded, the contrast agent that passed through the dural AVF showed multiple cortical venous refluxes and ectasia, with venous drainage into the left sigmoid sinus (Fig. 7A, B). In the MPR axial images of the 3D rotational CT performed after selecting the left ECA, the distal portion of the left transverse sinus connecting to the superior sagittal sinus was occluded, and the junction of the transverse sinus and the sigmoid sinus was extremely narrow. A venous pouch was visible in the transverse sinus between the occluded and narrowed sites. Most feeding arteries were connected to this pouch, and small, thin feeding arteries were also connected to the transverse sinus (Fig. 7C-E). If the shunted pouch was excluded and only the transverse sinus was blocked, it could recur as an inaccessible dural AVF with isolation observed on the follow-up examination because it was not appropriately treated. Therefore, the shunted pouch was blocked by selection. Angiography after approaching the vein with the left sigmoid sinus and selecting the transverse sinus after passing through the narrowed area with a microcatheter revealed that the microcatheter blocked the narrow area; therefore, the anatomy of the feeding artery and vein was more clear, and a shunted pouch that overlapped the sigmoid sinus was also visible (Fig. 7F). ONYX was injected after selecting the shunted pouch with a microcatheter. The shunted pouch and feeding arteries were then blocked. The remaining transverse sinus was selected using a new microcatheter, the remaining sinus lumen was blocked with a coil, and the procedure was completed (Fig. 7G). Angiography performed after the procedure revealed that the dural AVF was no longer visible, without recurrence at the 1-year follow-up angiography (Fig. 7H, I).
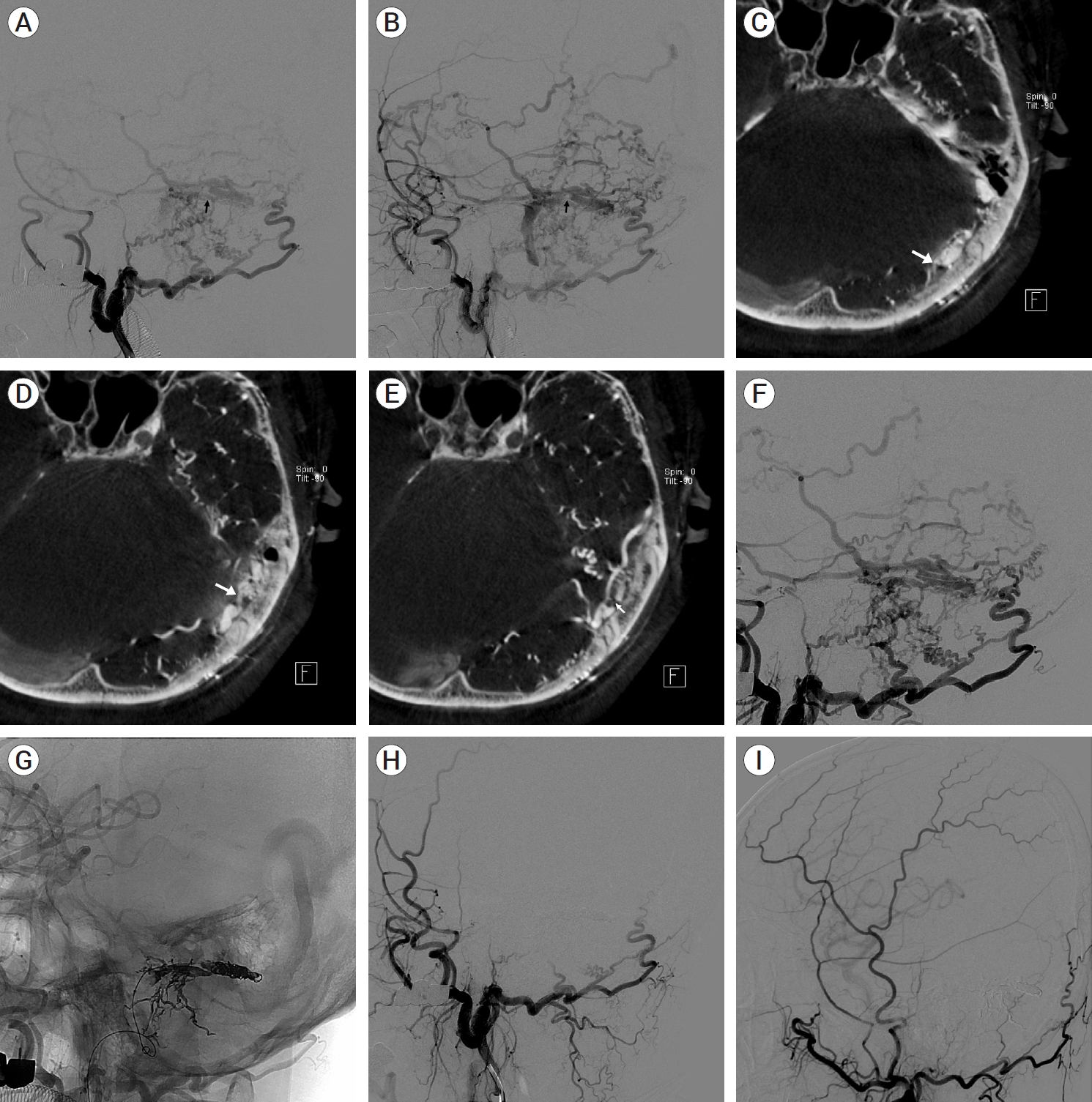
Case 3. Dural AVF in left transverse sinus and transvenous embolization. Left ECA selective angiography, lateral view in A and B. (A) Shunted pouch (black arrow). (B) Tight stenosis in junction of transverse and sigmoid sinus (black arrow). MPR images of 3D rotational CT in C, D, and E. (C) Occlusion in distal portion of transverse sinus (white arrow). (D) Shunted pouch (white arrow). (E) Tight stenosis in junction of transverse and sigmoid sinus (white arrow). (F) After passing through stenosis with microcatheter, stenosis was blocked by microcatheter. More detail of venous anatomy was visible. (G) Transvenous ONYX and coil embolization of dural AVF. (H) After treatment, immediately follow up angiography. Total occlusion of dural AVF. (I) 1 year follow up angiography, No recurrence of dural AVF. AVF, arteriovenous fistula; ECA, external carotid artery; MPR, multiplanar reconstruction; 3D, three-dimensional; CT, computed tomography
CONCLUSIONS
Understanding accurate venous anatomy is essential for treating dural AVFs. In dural AVFs, the vein is extremely tortuous and overlapping, making it challenging to identify using only AP and lateral projection images of digital subtraction angiography. By analyzing sectional images, such as MPR images of a 3D rotational CT and source images of MRA and CTA, accurate venous anatomy can be identified. If the venous anatomy is well understood and planned for treatment, it is possible to avoid unnecessary total sinus packing, with embolization of only the necessary site (shunted pouch), maintaining normal venous drainage as far as possible, thereby enabling fundamental treatment without complications.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.