Symptomatic perianeursymal cyst development 20 years after endovascular treatment of a ruptured giant aneurysm: Case report and updated review
Article information
Abstract
Perianeurysmal cysts are a rare and poorly understood finding in patients both with treated and untreated aneurysms. While the prior literature suggests that a minority of perianeurysmal cysts develop 1-4 years following endovascular aneurysm treatment, this updated review demonstrates that nearly half of perianeurysmal cysts were diagnosed following aneurysm coiling, with the other half diagnosed concurrently with an associated aneurysm prior to treatment. 64% of perianeurysmal cysts were surgically decompressed, with a 39% rate of recurrence requiring re-operation. We report a case of a 71-year-old woman who presented with vertigo and nausea and was found to have a 3.4 cm perianeurysmal cyst 20 years after initial endovascular coiling of a ruptured giant ophthalmic aneurysm. The cyst was treated with endoscopic fenestration followed by open fenestration upon recurrence. The case represents the longest latency from initial aneurysm treatment to cyst diagnosis reported in the literature and indicates that the diagnosis of perianeurysmal cyst should remain on the differential even decades after treatment. Based on a case discussion and updated literature review, this report highlights proposed etiologies of development and management strategies for a challenging lesion.
INTRODUCTION
Perianeurysmal cysts are a rare entity that have been associated with both treated and untreated intracranial aneurysms. Over the past three decades, only a few cases have been reported in the English-language literature. Our case and the prior reports highlight diversity in cyst presentation, treatment of the associated aneurysms, and the management of the cysts.
In the current review, perianeurysmal cysts were decompressed if deemed symptomatic. The most common presenting symptoms were headache in 40% of patients (n=11/27), and weakness or gait difficulty in 29.6% (n=8). 64.3% (n=18/28) underwent cyst decompression, and of these 38.9% (n=7/18) underwent endoscopic or stereotactic fenestration as the initial cyst intervention (Table 1). Recurrences were common at 38.9% (n=7/18) and occurred mostly between 1-3 months, with one report of recurrence at 5 years. Recurrences were distributed between open and endoscopically-treated groups, and were managed with both surgical and adjuvant approaches (Table 2). Friedman et al. describe the placement of a cysto-peritoneal shunt after multiple cyst fenestrations and re-accumulations; ultimately, the cyst reaccumulated despite shunting but was no longer symptomatic [4]. Grandhi et al. describe cyst recurrence after stereotactic aspiration, treated with re-aspiration and irradiation [6].
Nearly half of the perianeurysmal cysts were diagnosed following endovascular aneurysm treatment (n=13/28) [1,4-6,9,10,12-14,18,19]. Notably, the remainder of were diagnosed concurrently with an associated aneurysm (n=15/28); none were diagnosed post-clipping [2,3,7,8,11,15-17]. This is an update to a recent review demonstrating that the majority (n=14/19) of perianeurysmal cysts were diagnosed simultaneously with an associated aneurysm rather than following treatment [12]. 25.9% (n=7/27) of patients had prior aneurysm rupture; all were treated endovascularly. Of those who underwent endovascular intervention (n=15), the majority (n=11) experienced aneurysm residual or recurrence requiring more than one treatment. 40% (n=6/15) underwent both coiling and stenting, and embolization with bare platinum coils was most common (Table 1).
Here, we report a case of symptomatic perianeurysmal cyst diagnosed 20 years following initial coiling of a ruptured ophthalmic artery aneurysm. The patient underwent stereotactic aspiration with cyst reaccumulation over the course of one month, followed by craniotomy for fenestration. This case represents the longest latency from initial aneurysm coiling to cyst diagnosis reported to date, highlights the unclear relationship with endovascular aneurysm treatment, and reiterates the challenges faced in managing a recurrent cyst.
METHODS
For the case report, informed consent was obtained from the patient. The complete history was obtained via retrospective chart review.
For the review, a search of PubMed and Embase databases was performed using combinations of MeSH and key terms to include all relevant full-text publications through November 2022. Key terms included “perianeurysmal,” “cerebral aneurysm,” “intracranial aneurysm,” and “cyst” searched with both ‘AND’ and ‘OR’ configurations. Individual article reference lists were also screened for additional publications missed on the initial search.
CASE DESCRIPTION
The patient is a 71-year-old woman with type II diabetes, hypertension, and heart failure who first presented in 2002 with 1 week of headaches due to subarachnoid hemorrhage and a ruptured 2.8 cm right ophthalmic aneurysm. The aneurysm was embolized with 56 Guglielmi detachable coils (GDC) and Micrus coils. Over the next year, the aneurysm developed significant coil compaction with a 2 cm opacifying lumen and 4-5 mm neck, requiring two re-embolizations. Ten months after the initial embolization, a Neuroform stent was deployed across the aneurysm neck and 18 GDC and Micrus coils were placed; at 12 months, 5 additional Micrus coils were placed. The patient also had unruptured 5 mm left and 6 mm right intracranial carotid artery aneurysms that were electively embolized in 2003. Multiple follow-up angiograms through 2016 demonstrated stability of the treated aneurysms. No overt cystic lesions were seen on brain MRIs through 2014 or head CTs through 2021.
In January 2022, the patient presented to the hospital experiencing vertigo and nausea for one week. She was otherwise neurologically intact and denied headache or emesis. MRI demonstrated a 3.4 cm right frontal cystic lesion in the caudate head adjacent to the coiled ophthalmic artery aneurysm, with 10 mm midline shift (Fig. 1). The post-contrast sequence also demonstrated nodular enhancement along the periphery of the coil mass. A diagnostic cerebral angiogram demonstrated stable occlusion of the right ophthalmic aneurysm without blood flow into the cyst. While the differential diagnosis included primary brain tumors or vascular lesions, the apposition of the cyst to the aneurysm as well as its lack of blood flow were highly suggestive of perianeurysmal cyst.
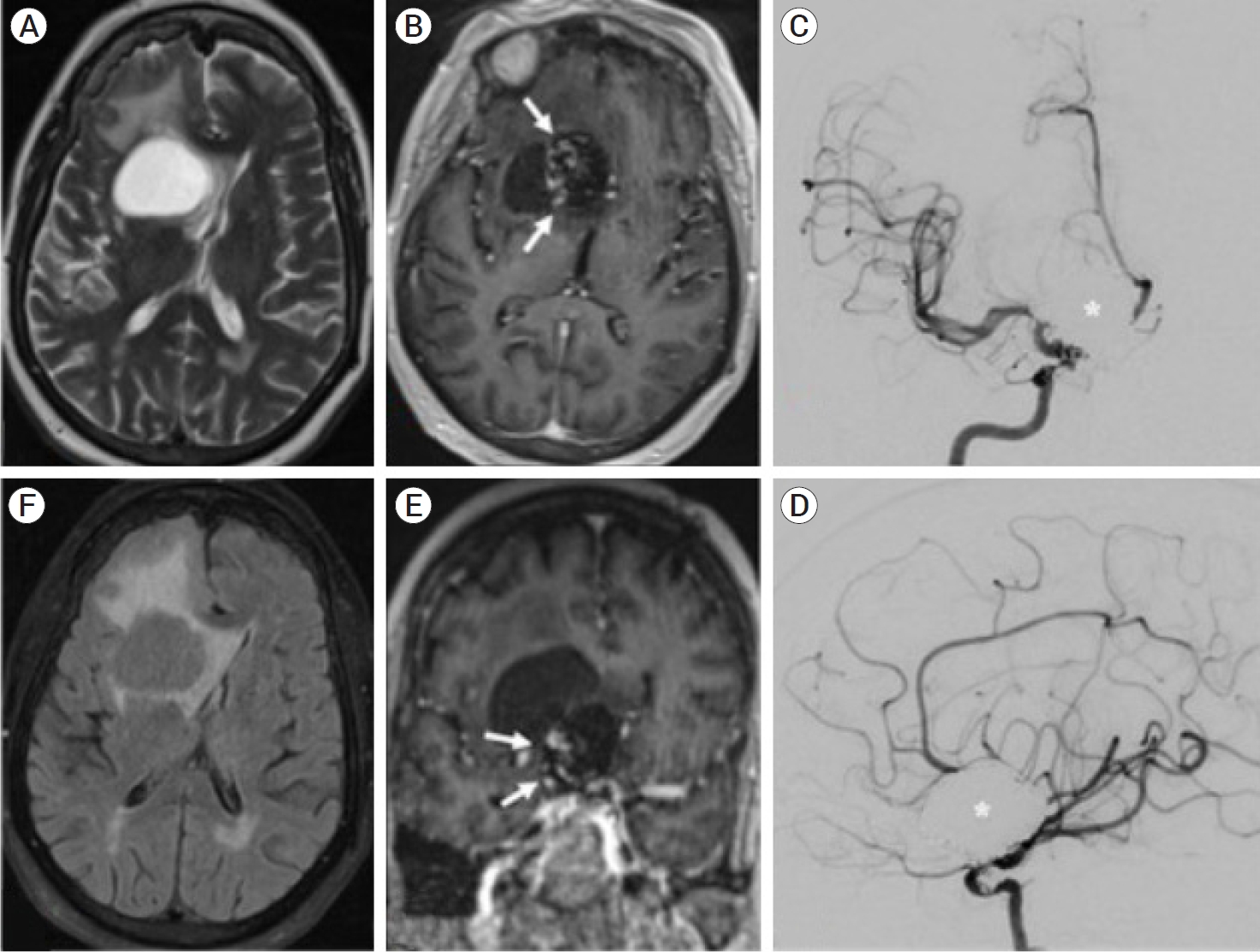
(A) T2-weighted magnetic resonance image (MRI) demonstrates a cystic-appearing lesion within the right caudate head causing significant regional mass effect and extensive vasogenic edema. (B, E) Post-contrast T1-weighted MRI with abnormal nodular enhancement (white arrows) along the lateral margin of a previously coiled giant right ICA terminus aneurysm. (C, D) Digital subtraction angiography confirms complete occlusion of a giant right ICA terminus aneurysm (white asterisk). (F) T2-FLAIR MRI imaging shows incomplete signal suppression within the right caudate head lesion suggestive of a complex cyst with proteinaceous debris. ICA, internal carotid artery
The initial procedure of choice was stereotactic fenestration which is a minimally invasive, low risk, and brief procedure that would address the mass effect and potentially obviate the need for a more invasive craniotomy. The patient’s history of heart failure and diabetes also supported a trial of the minimally invasive option. Lastly, given the frontal location of the lesion and the patient’s presentation without neurologic deficits, monitoring for re-accumulation with surveillance scans would be a safe option. She underwent a right frontal burr hole for stereotactic drainage of the cyst, from which 21 ccs of yellow fluid were aspirated without resistance. The cyst fluid coagulated within the sample tube. Cytology confirmed proteinaceous and acellular material. A post-op head CT demonstrated collapsed cyst. She remained neurologically intact and was discharged two days later.
A surveillance MRI on post-op day 22 demonstrated that the cyst had reaccumulated to its prior size. Due to reaccumulation and mass effect, on post-op day 35 she underwent a right frontotemporal craniotomy for cyst fenestration. The cyst was accessed via a transcortical approach, and yellow fluid was drained. Under the operating microscope, the cyst wall contained numerous small blood vessels and was gently coagulated. A post-op CT again demonstrated collapsed cyst. On outpatient follow-up, a CT on post-craniotomy day 27 demonstrated no cyst reaccumulation. The patient expressed her satisfaction with her treatment and resolution of symptoms.
DISCUSSION
We describe a 3.4 cm perianeurysmal cyst diagnosed 20 years after the initial embolization of a ruptured 2.3 cm ophthalmic aneurysm, which eventually required two additional embolizations. Our case falls under the group of perianeurysmal cysts that develop after endovascular aneurysm treatment, a trend that has not been previously reported. Moreover, our case demonstrates the longest latency from coiling to definitive cyst diagnosis. Along with our review of the literature, our current case suggests that either mechanical or exudative/secretory forces play a dominant role in cyst formation.
After consideration of open craniotomy for resection of cyst wall, ultimately our case underwent stereotactic fenestration as the initial method of cyst decompression. The literature suggests that cyst recurrence is common after simple fenestration, regardless of open, endoscopic, or stereotactic approach. As discussed above, we determined our initial approach given the minimally-invasive nature of stereotactic fenestration, our patient’s medical history, and the cyst’s in an easily accessible and non-eloquent area. In a different scenario of a healthier patient presenting with neurologic deficits in a less stereotactically-favorable location, perhaps proceeding with open cyst wall resection would be the most appropriate treatment option [4,6].
As illustrated by the current case, perianeurysmal cyst recurrence after initial fenestration presents a management challenge. Along with most other cases of cyst recurrence, we performed a second, more extensive fenestration via open craniotomy to provide diversion of cyst fluid. Three cases describe fenestration into a ventricle either endoscopically or via craniotomy; only one case required a further fenestration [6,10,13]. A less invasive option is chronic drainage via cysto-peritoneal shunt or Ommaya, although the risk of shunt occlusion is not trivial given the typically highly proteinaceous cyst fluid; Friedman et al. report (asymptomatic) cyst recurrence despite shunting [4,9]. The second treatment strategy involves reduction in cyst fluid production, which may be accomplished by irradiation or gentle cyst wall coagulation as performed in the current case [6]. Another escalation described by König et al. entails resection of the associated aneurysm; this procedure may also prevent cyst accumulation, although for unclear reasons [10].
One additional challenge in managing perianeurysmal cysts is that the mechanism of fluid accumulation remains unclear. Below, we highlight possible relationships between endovascular aneurysm treatment and cyst formation [10,12]. Broadly, potential etiologies include 1) mechanical pressure from aneurysm or coil mass, 2) infection or inflammation, 3) gliosis of adjacent brain following initial hemorrhage, or 4) coil extrusion.
One theory posits that mechanical pressure exerted by aneurysm pulsations damages adjacent parenchyma which eventually undergoes cystic degeneration [12]. The majority of endovascularly-treated aneurysms required multiple treatments for recurrence or residual. Friedman et al. posit that a coiled aneurysm then becomes a semirigid mass which transmits pulsatile pressure waves [4]. A larger coil mass could conceivably exert greater force on the surrounding brain parenchyma leading to greater parenchymal degeneration, potentially over decades. There was no obvious relationship between number of recurrences or endovascular treatments and cyst size. While this mechanism is plausible considering the current case report, more data are needed to establish clear relationships between coil mass, cyst size, and latency to cyst development.
Another proposed mechanism is that cyst formation is due to direct exudation from the aneurysm wall, secondary to aberrant angiogenesis or increased inflammation [12,17]. Histologic examination of perianeurysmal cysts have demonstrated inflammatory infiltrates and reactive gliosis, with thrombosed aneurysm or foreign body deposition as possible inciting factors [2,12]. The majority patients who underwent endovascular aneurysm treatment received bare metal coils. In a case embolized with bare platinum coils with possible chronic erosion of coil material through the aneurysm wall, König et al. report cyst histology with eosinophilic granular bodies [10]. As with other reported cases, the highly proteinaceous cyst fluid in the current case supports an exudative process, and the many small vessels within the cyst wall could be consistent with both aberrant angiogenesis and inflammation. Regarding the current case, although it would be unusual to develop a reaction to the coil mass over a decade after its initial deposition, the inflammatory response is likely multifactorial.
König et al. report a recurrent cyst that abates only after resection of the coiled aneurysm [10]. Of the patients who underwent upfront aneurysm clipping and concurrent open cyst fenestration (including 3 who had resection of part or all of the aneurysm), none experienced cyst recurrence. This suggests that factors from the aneurysm contribute not only to the initial development but also to the maintenance of the cyst, and that clipping may differentially mitigate these factors.
CONCLUSIONS
As perianeurysmal cysts are rare finding, much about their etiology and management remain poorly understood. This report highlights the development of perianeurysmal cysts following aneurysm coiling and the possibility of cyst development decades after treatment. Recurrent cysts present a management challenge, and were treated with repeat decompression, cysto-peritoneal shunts and reservoir implantations, and radiation. While prospective studies may be limited by the rarity of these lesions, future directions include trending cysts at lengthier follow-up intervals, more detailed histologic studies of cyst wall biopsies, and modeling the effects of pressure exerted by aneurysms or coil masses on surrounding parenchyma.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.