 |
 |
| J Cerebrovasc Endovasc Neurosurg > Epub ahead of print |
Abstract
Fibromuscular dysplasia (FMD) is a noninflammatory arterial diseases that affects predominantly women. Multiple studies have demonstrated an increased prevalence of FMD in patients who experience carotid or vertebral artery dissection (VAD). This case report presents a 57-year-old female who presented with a headache and was diagnosed with partially thrombosed giant aneurysm of vertebral artery. This aneurysm was successfully treated with flow-diverter and coil, but new onset rupture of vertebral artery was detected two weeks later, leading to internal trapping. This case report underscores the need for awareness and understanding of treatment of dissection and aneurysm in patient who is suspected FMD
Fibromuscular dysplasia (FMD) is an idiopathic, segmental, nonatherosclerotic, and noninflammatory disease of small- to medium-sized arteries, characterized by disrupted architecture of the vessel wall [9]. Although FMD is systemic disease affecting more than one vascular district in a single patient, the involvement of the cervical (carotid and vertebral) arteries is far more frequent than that of other vessels [12]. Multiple studies has been reported that cerebrovascular FMD has been associated to vertebral artery dissection (VAD) and aneurysm, the most frequent cause of stroke in young and middle aged adults.
VAD is considered an uncommon but increasingly recognized cause of subarachnoid hemorrhage (SAH) and brainstem ischemia [2,8]. Endovascular intervention such as flow-diverter is good option for VAD, providing vascular reconstruction and thrombosis of aneurysmal sac when it exists [8]. But, natural history and optimal treatment of VAD with FMD is unclear. Here, we describe fatal rupture of vertebral artery after flow-diversion stenting in FMD patients.
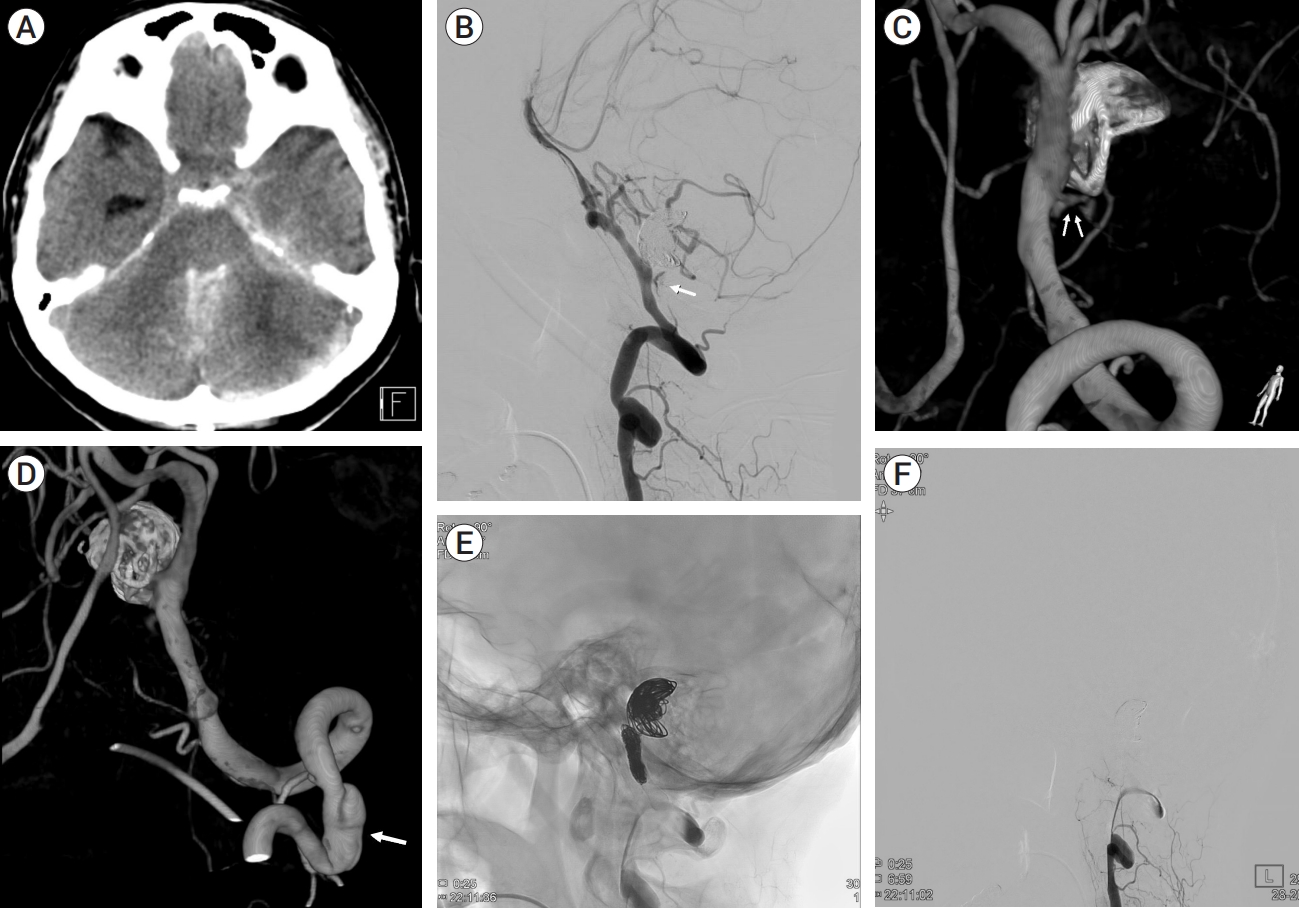
A 57-year-old female was admitted to emergency room for the headache. The patient had no significant past medical history but complained of chronic headache. In neurological examination, there was no neurological deficit. In radiological examination, brain computed tomography (CT) demonstrated 25 mm sized hyperdense mass in the posterior fossa (Fig. 1A). There was no SAH or acute brain lesion. CT angiography (CTA) showed 16 mm sized aneurysm at left vertebral artery (VA) (Fig. 1B). To determine the treatment method and timing, vessel wall MRI was performed. Vessel wall MRI revealed 25 mm sized partially thrombosed aneurysm and enhancement of the aneurysm wall, leading to suspicion of unstable aneurysm (Fig. 2A, B). In digital subtraction angiography (DSA), a 16 mm sized aneurysm was identified in the left VA, and the string beads appearance was revealed in the right cervical ICA that raised suspicions of FMD (Fig. 3A, B). After antiplatelet loading, the giant partially thrombosed aneurysm was treated with DERIVOŌōć (Acandis, Germany) flow-diverter and coil. (Fig. 3C, D). The patient was discharged without any problem. Two weeks after the procedure, the patient visited to the emergency room with a decreased level of consciousness, and brain CT revealed thick SAH, intraventricular hemorrhage in the posterior fossa and temporal horn enlargement which is indicated hydrocephalus (Fig. 4A). Because the mental status was semicoma, external ventricular drain (EVD) was placed immediately and DSA was performed. In DSA, contrast leakage due to VAD was observed just proximal to the treated aneurysm. Previously treated aneurysm was found to be occluded (Fig. 4B, C). Additionally, new onset artery dissection at C3 level had been confirmed that was not previously observed (Fig. 4D). During the procedure, there was no catheter-induced injury associated with this lesion. Initially, stent insertion was performed in the dissection area. However, contrast medium was leaked continuously. The patientŌĆÖs contralateral VA flow was intact, we performed internal trapping at the dissecting area. After trapping, no contrast medium leakage was observed (Fig. 4E, F). Because severe cerebellar edema was observed in follow up brain CT, decompressive suboccipital craniectomy was performed. Despite the surgery, the patient continued to deteriorate. The family withdrew care and the patient expired on the 10th day after the rupture of vertebral artery.
FMD was first described as fibromuscular hyperplasia in patients with renal stenosis. However, subsequent pathological classifications used the term FMD. Histologically, FMD lesions are heterogeneous, with various degrees of collagen hyperplasia, internal elastic lamina rupture, and disorganization of the media layer [11]. But, pathological specimens are rarely obtained, the diagnosis of FMD is most often made on the basis of a characteristic angiographic appearance such as multiple stenoses and the string of beads appearance. In earlier reports of consecutive angiograms performed in imaging department, the prevalence of FMD ranged from 0.3% to 3.2% [11]. Cerebrovascular FMD (C-FMD) typically involves the extracranial portion of the carotid and vertebral arteries [4]. Lather et al. [5] reported that prevalence of intracranial aneurysm among women with FMD was 12.9%, significantly greater than that estimated for the general population of women. However, the diagnosis of FMD can be challenging in case of acute dissection, as angiographic features of both diseases can be similar. Talarowska et al. [10], there was no distinctive discriminating factors between patients with VAD with and without FMD. Thus, FMD should be suspected in all patients with VAD. Cross-sectional imaging of arteries of the head, neck, chest, abdomen, and pelvis with CTA or MRA to evaluate the lesion that associated with FMD.
Optimal treatment for artery dissection with FMD is controversial because of poor knowledge of the natural history and the lack of randomized trials that compared the different treatment options [11]. Therefore, the treatment is essentially the same as that in patients without FMD who develop dissection. For endovascular intervention, internal trapping and stent with or without coil can be considered. Li et al. [6] reviewed 65 patients with unruptured VAD aneurysm who underwent endovascular treatment. Among them, patients who underwent internal trapping exhibited more ischemic symptoms compared to those in the stent-assisted coiling group. Clinical outcome was similar between the two groups. Recurrence rates was higher in stent-assisted coiling group than internal trapping group, although this difference did not reach statistical significance. Jin et al. [3] reviewed 13 patients with unruptured VAD aneurysms who trapping and stent-assisted coil embolization were performed. There were no significant differences in complications or clinical outcomes between the two groups. But, internal trapping can prevent retrograde flow form the contralateral vertebral or basilar arteries into the dissecting aneurysm, effectively precluding aneurysm re-expansion [7,12]. Zhao et al. [12] suggested that high wall shear stresses (WSS) may play critical roles in recurrence of VAD after stent insertion. We suggested the hypothesis that the fragile vessel wall may be more vulnerable to the WSS occurring after stent insertion in FMD patients, leading to a higher recurrence rate. De Bray et al. [1] reviewed 103 patients who had been admitted for a cervical artery dissection. Five patients had dissection recurrence and four of these 5 patients diagnosed FMD. This study suggested that patients with cervical artery dissection have a higher risk of recurrent dissection when associated with FMD. Therefore, if patient is suspected of having FMD, considering a more aggressive treatment like internal trapping if possible, rather than stent-assisted coiling may be more viable option.
FMD patients with dissection have a higher risk of recurrence. As demonstrated in our case, due to the fragile vessel wall in FMD patients, artery dissection can occur even after stent insertion. Therefore, the author suggests that more definitive treatment options such as internal trapping are considered to prevent recurrence or fatal hemorrhage in FMD patients with aneurysm or dissection.
Fig.┬Ā1.
(A) CT shows 25 mm sized hyperdense lesion at posterior fossa. (B) CTA showed 16 mm aneurysm at left vertebral artery. CT, computed tomography; CTA, CT angiography
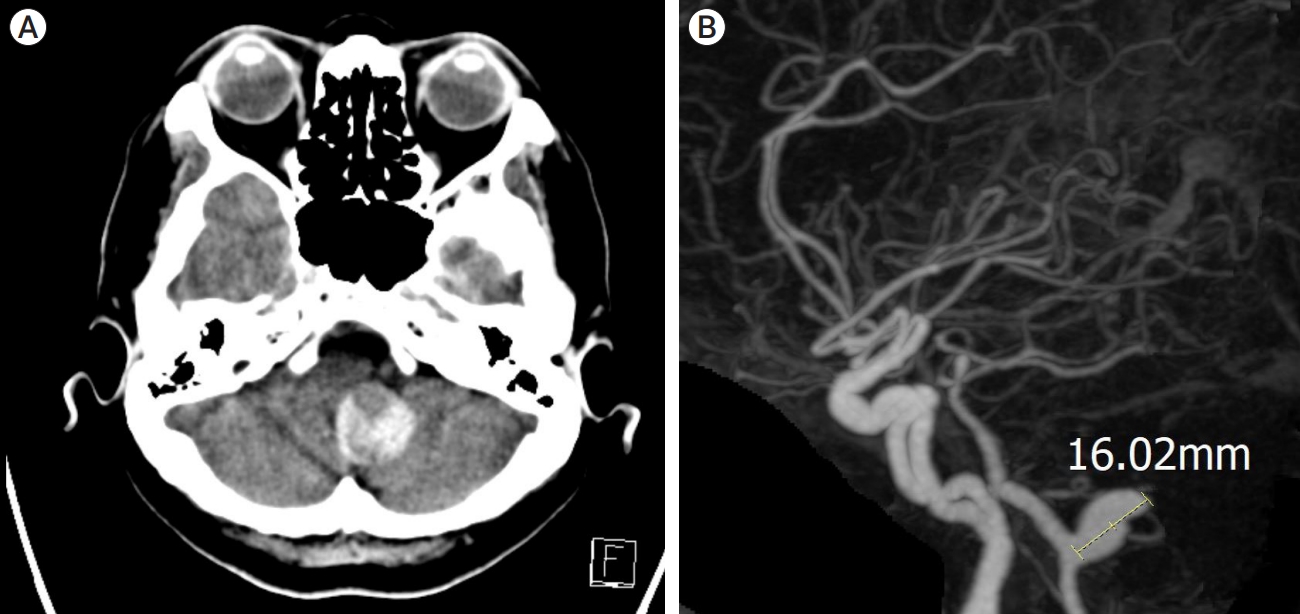
Fig.┬Ā2.
Vessel wall MRI shows 25 mm giant thrombosed aneurysm and enhanced aneurysm wall. (A) Vessel wall MRI, axial view. (B) Vessel wall MRI, sagittal view.
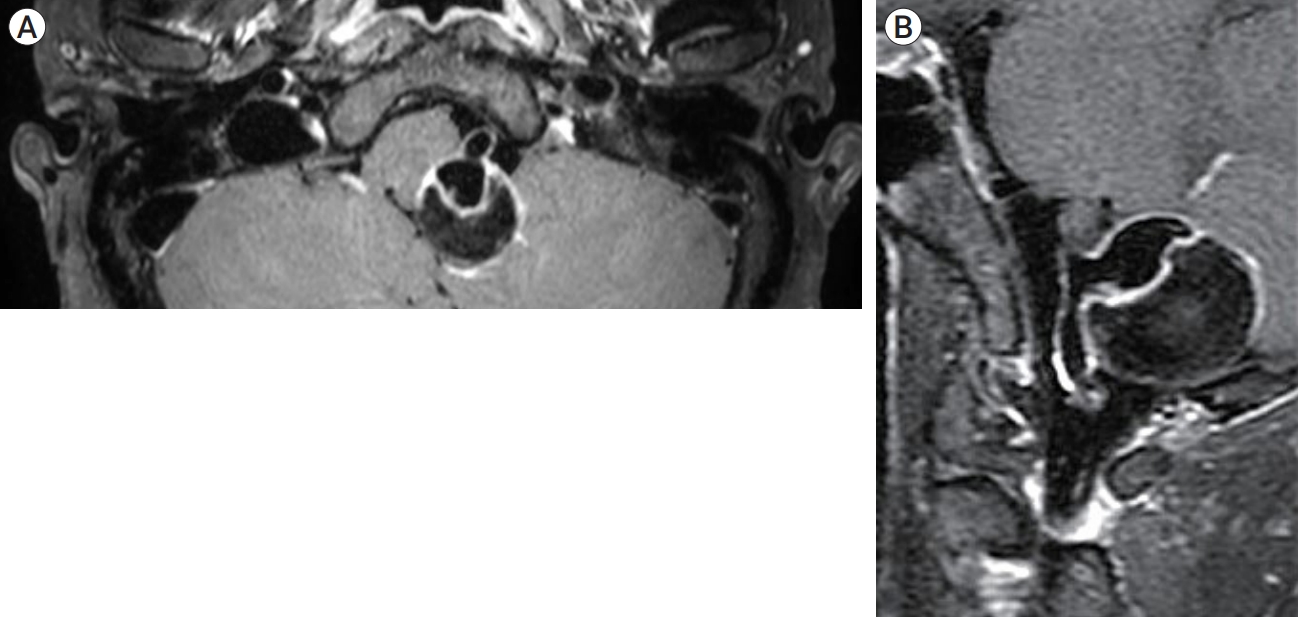
Fig.┬Ā3.
(A) DSA shows aneurysm at left VA. (B) DSA shows the string of beads appearance that represented FMD (white arrow) (C) Fluoroscopy shows flow-diverter (white arrow) with coil embolization (white arrowhead). (D) DSA shows contrast stagnation after flow-diverter insertion and coil embolization. DSA, digital subtraction angiography; VA, vertebral artery; FMD, fibromuscular dysplasia

Fig.┬Ā4.
(A) CT shows SAH, IVH at posterior fossa and temporal horn widening, indicating hydrocephalus. (B) DSA shows the contrast leakage (white arrow). (C) 3D DSA shows contrast leakage at the site of VA dissection (double white arrows). (D) 3D DSA shows new onset artery dissection at the C3 level that was not previously observed (white arrow). (E) Fluoroscopy shows endovascular internal trapping of left VA. (F) DSA shows totally occluded dissected VA segment. CT, computed tomography; SAH, subarachnoid hemorrhage; IVH, intraventricular hemorrhage; DSA, digital subtraction angiography; VA, vertebral artery
REFERENCES
1. De Bray JM, Marc G, Pautot V, Vielle B, Pasco A, Lhoste P, et al. Fibromuscular dysplasia may herald symptomatic recurrence of cervical artery dissection. Cerebrovasc Dis. 2007 23(5-6):448-52.



2. Iihara K, Sakai N, Murao K, Sakai H, Higashi T, Kogure S, et al. Dissecting aneurysms of the vertebral artery: A management strategy. J Neurosurg. 2002 Aug;97(2):259-67.


3. Jin SC, Kwon DH, Choi CG, Ahn JS, Kwun BD. Endovascular strategies for vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2009 Sep;30(8):1518-23.



4. Kesav P, Manesh Raj D, John S. Cerebrovascular fibromuscular dysplasia - A practical review. Vasc Health Risk Manag. 2023 Aug;19:543-56.




5. Lather HD, Gornik HL, Olin JW, Gu X, Heidt ST, et al. Prevalence of intracranial aneurysm in women with fibromuscular dysplasia: A report from the US registry for fibromuscular dysplasia. JAMA Neurol. 2017 Sep;74(9):1081-7.



6. Li H, Li XF, Zhang X, He XY, Duan CZ, Liu YC. Treatment of unruptured vertebral dissecting aneurysms: internal trapping or stent-assisted coiling. Int J Neurosci. 2016 126(3):243-8.


7. Mehta B, Burke T, Kole M, Bydon A, Seyfried D, Malik G. Stent-within-a-stent technique for the treatment of dissecting vertebral artery aneurysms. AJNR Am J Neuroradiol. 2003 Oct;24(9):1814-8.


8. Narata AP, Yilmaz H, Schaller K, Lovblad KO, Pereira VM. Flow-diverting stent for ruptured intracranial dissecting aneurysm of vertebral artery. Neurosurgery. 2012 Apr;70(4):982-8.



9. Olin JW, Gornik HL, Bacharach JM, Biller J, Fine LJ, Gray BH, et al. Fibromuscular dysplasia: State of the science and critical unanswered questions: A scientific statement from the american heart association. Circulation. 2014 Mar;129(9):1048-78.


10. Talarowska P, Dobrowolski P, Klisiewicz A, Kostera-Pruszczyk A, Członkowska A, Kurkowska-Jastrzębska I, et al. High incidence and clinical characteristics of fibromuscular dysplasia in patients with spontaneous cervical artery dissection: The ARCADIA-POL study. Vasc Med. 2019 Apr;24(2):112-9.



11. Touz├® E, Oppenheim C, Trystram D, Nokam G, Pasquini M, Alamowitch S, et al. Fibromuscular dysplasia of cervical and intracranial arteries. Int J stroke. 2010 Aug;5(4):296-305.



12. Zhao KJ, Zhao R, Huang QH, Xu Y, Hong B, Fang YB, et al. The interaction between stent(s) implantation, PICA involvement, and immediate occlusion degree affect symptomatic intracranial spontaneous vertebral artery dissection aneurysm (sis-VADA) recurrence after reconstructive treatment with Stent(s)-Assisted Coiling. Eur Radiol. 2014 Sep;24(9):2088-96.




-
METRICS

-
- 0 Crossref
- 0 Scopus
- 544 View
- 28 Download
- ORCID iDs
-
Dae-Won Kim

https://orcid.org/0000-0003-2151-2841 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



