Two consecutive ruptured intracranial aneurysm in patient with multiple intracranial aneurysms
Article information
Abstract
When aneurysmal subarachnoid hemorrhage due to multiple aneurysms is suspected, identifying the rupture site is essential to determine the exact surgical site, but it may not be easy. Even if embolization is adequately performed, complications may remain. Typical complications include rebleeding and hydrocephalus in the early phase and delayed cerebral ischemia in the delayed phase. Herein, we describe a case of rupture of an intracranial aneurysm after performing embolization for a different ruptured intracranial aneurysm in a patient with multiple intracranial aneurysms. Patients with multiple intracranial aneurysms need to be considered for closer observation than those with a single ruptured intracranial aneurysm, even if the patient’s prognosis is good.
INTRODUCTION
The mortality rate of ruptured aneurysmal subarachnoid hemorrhage (SAH) is over 35% [3], and is a severe disease which often leaves severe neurological sequelae even if patients survive. The risk of rebleeding is high within 24 h of onset, particularly within 8 h, and the prognosis is extremely poor when rebleeding occurs, indicating that immediate diagnosis and appropriate treatment are essential factors for the patient’s prognosis [5].
If brain computed tomography (CT) performed when the patient visits the hospital shows SAH findings and a ruptured aneurysm is strongly suspected in clinical symptoms, it is common to perform CT angiography (CTA) or magnetic resonance angiography to identify the rupture site. Digital subtraction angiography (DSA) is then performed to establish a plan for adequate surgical treatment [1].
However, a study found that 26–33% of all aneurysm patients have multiple aneurysms [6]. In patients with multiple aneurysms, accurate diagnosis of the rupture site by performing the aforementioned examinations is not easy compared to general cases [4].
We describe a patient with multiple intracranial aneurysms who underwent coil embolization, but one of the aneurysms, which was initially determined not to have ruptured, was bleeding. All procedures described below were performed with a digital angiography system (Allura® Xper FD20C, Philips Healthcare, Best, Netherlands).
CASE DESCRIPTION
A 60-year-old woman who presented with drowsy consciousness was referred to our hospital. An initial non-enhanced axial brain CT scan revealed diffuse SAH in the right frontotemporal region and basal cisterns, graded 1 according to the modified Fisher scale. The patient presented with a grade of 3 according to the Hunt and Hess scale. Three-dimensional (3D)-CTA was performed following the CT scan, 2 h after the initial attack. A 1-mm aneurysm in the right middle cerebral artery (MCA) bifurcation and a 4-mm irregular aneurysm in the right proximal A1 were identified on CTA. Based on the size and shape, the A1 aneurysm was considered the source of bleeding (Fig. 1).
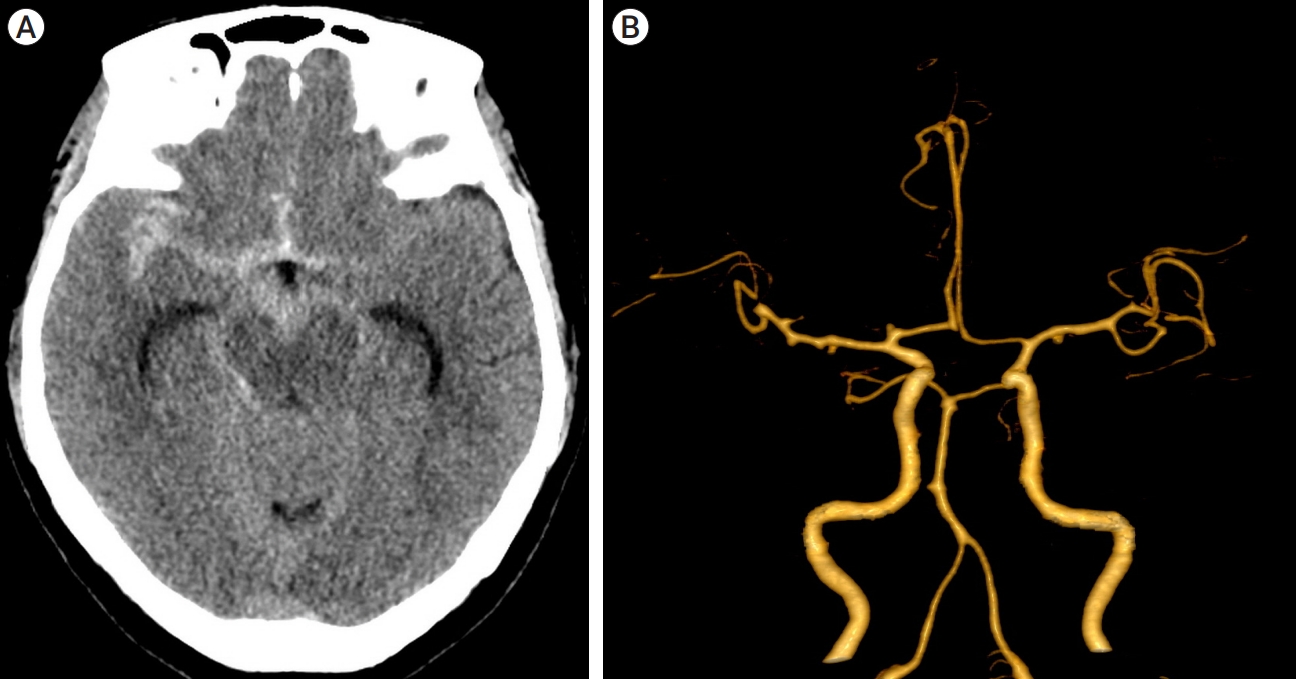
Initial radiographic findings. (A) CT scan showing subarachnoid hemorrhage in the basal cistern. (B) CT angiography showing an aneurysm arising from the proximal A1 segment. CT, computed tomography
First intervention
DSA identified a ruptured aneurysm on the right A1 portion. Emergency stent-assisted coil embolization was carried out. After completion of aneurysm coiling, no remnant aneurysm sac was visible (Fig. 2). Postoperative brain perfusion CT demonstrated no change of diffuse SAH and minimal intraventricular hemorrhage in both lateral ventricles. The patient was monitored in the neurosurgical intensive care unit and received dual antiplatelet medications (aspirin and clopidogrel).

DSA before and after endovascular treatment. (A) Anteroposterior view of the digital subtraction angiography showing an aneurysm arising from the proximal A1 segment. It is irregular shape of aneurysm with height of 3.66 mm and transverse diameter is 1.68 mm, with a neck size of 3.04 mm. (B) Oblique view of the digital subtraction angiography showing an aneurysm arising from the proximal A1 segment. (C) Anterolateral working view of the digital subtraction angiography suggests the presence an aneurysm from right distal M1 segment. (D) Postoperative DSA. Red colored area indicates the embolization materials (Coil). DSA, digital subtraction angiography
Follow-up
On postoperative day 7, the patient recovered with alert mentality and demonstrated no sequelae. Follow-up DSA demonstrated no change in either the A1 or MCA bifurcation aneurysm (Fig. 3), so the patient was transferred to a general ward. On postoperative day 17, unenhanced brain CT demonstrated newly noted 6-cc intracranial hemorrhage (ICH) in the right frontotemporal lobe and minimal brain midline shifting to left. The SAH in the right sylvian fissure was slightly decreased. Based on the previous history of SAH and lack of neurologic deterioration, the ICH was considered to be hematoma expansion due to resolution. Clopidogrel was discontinued.
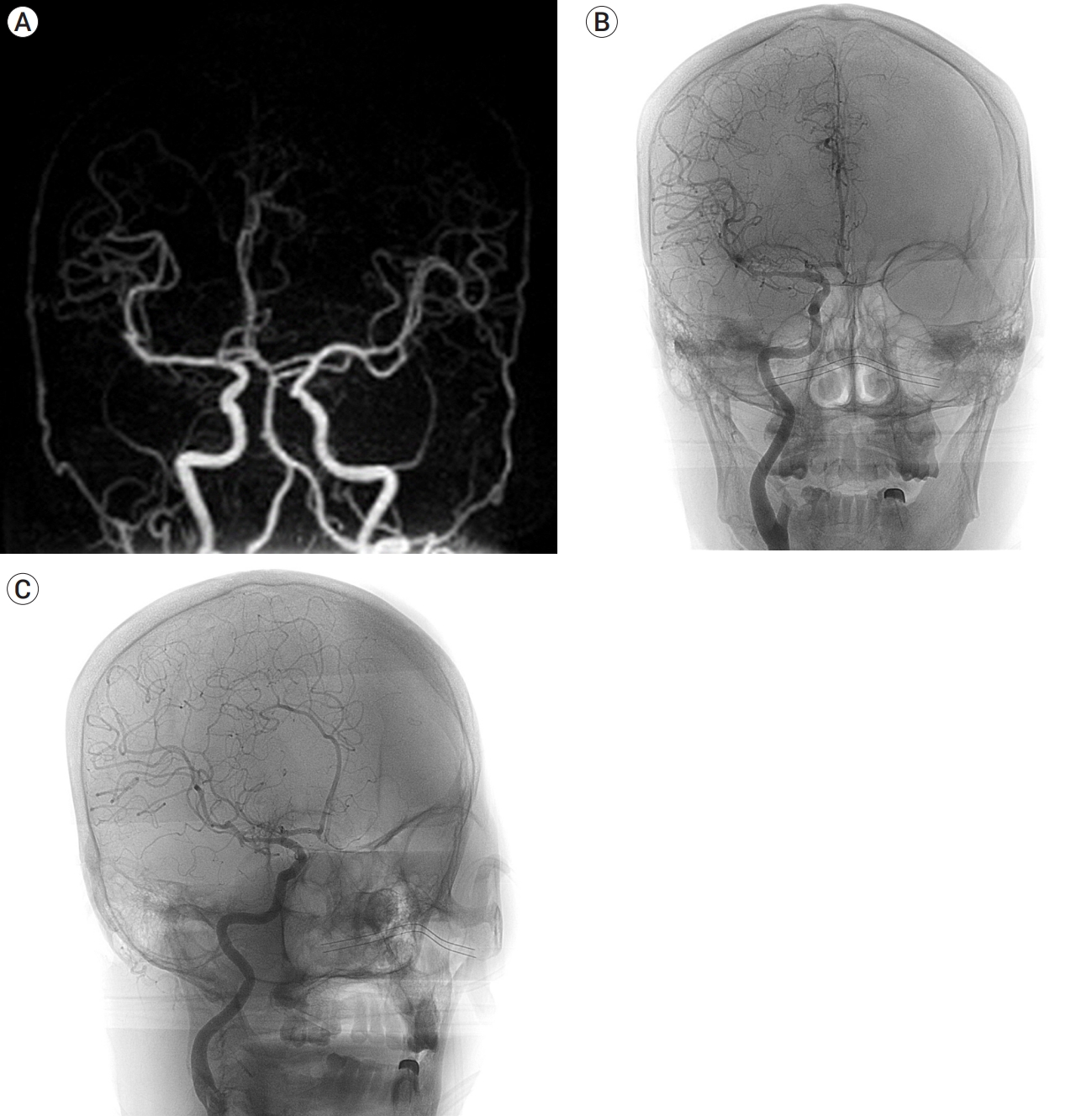
(A) MRA on the third day after endovascular treatment. It showed that there was no significant change in aneurysm in both aneurysms. (B and C) DSA on the 7th day after endovascular treatment. It showed that there was no significant change in both aneurysms. Anteroposterior view (B) and working view (C). DSA, digital subtraction angiography
Second presentation and intervention
On postoperative day 23, the patient suddenly fell into a semi-coma mentality. Unenhanced brain CT demonstrated 30 cc of ICH in the right frontotemporal lobe and 3D-CTA demonstrated a pseudoaneurysm on the right MCA bifurcation, probably due to ruptured aneurysm in the right distal MCA bifurcation portion, and irregular narrowing of the right middle cerebral artery (MCA), probably due to vasospasm (Fig. 4). Through DSA, we identified a pseudoaneurysm on the right MCA bifurcation. We performed coil embolization on the ruptured aneurysm, decompressive craniectomy with duroplasty and stereotactic extraventricular drainage catheter insertion, and ICH drainage catheter insertion. Postoperative brain perfusion CT demonstrated a perfusion defect in the right frontotemporal region. 46 days after secondary intervention, the patient was transfer to rehabilitation department. The patient recovered with semi coma mentality. Considering patient’s vegetative state, tracheostomy and gastrostomy was done.

CT and DSA on the 23th day after endovascular treatment, immediately after sudden mental change. (A) CT scan showing intracerebral hemorrhage in the right frontal lobe. (B) CT angiography showing a newly noted 14 mm aneurysm in the right distal M1 portion with Irregular narrowing of the right MCA. (C) Postoperative DSA. Red colored area indicates the embolization materials (Coil). CT, computed tomography; DSA, digital subtraction angiography
DISCUSSION
Early intracranial aneurysm surgery after SAH is essential to prevent rebleeding and effectively treat delayed vasospasm. Thus, identifying the rupture site is mandatory to determine the accurate surgical site for SAH in patients with multiple intracranial aneurysms [5]. Various methods, including clinical features such as focal neurological symptoms, CT, CTA, magnetic resonance imaging, magnetic resonance angiography, and cerebral angiography, can be used to determine the accurate rupture site [7].
According to the diagnosis flow chart for determining the initial rupture site proposed by Nehls et al., the rupture site is highly likely to be one of the following three cases; when diffuse SAH or local hematoma is present on CT findings and a single aneurysm is identified on cerebral angiography; when two or more aneurysms are present on cerebral angiography and local hematoma is in the same area on CT, or the most irregularly shaped or largest aneurysm is considered to be bleeding if no local hematoma is identified; and when irregularity of shape is a more crucial criterion than the size of the aneurysm, of which the daughter loculus is the most critical criterion for determining the rupture site [2].
In this case, stent assisted coiling was performed after determining the right A1 aneurysm as the rupture site based on size and irregularity. On postoperative day 23, the patient was found lying on the toilet in a semi-coma condition, and it was determined that the MCA bifurcation aneurysm, which was initially determined not to be ruptured, was ruptured according to CTA and cerebral angiography.
Postoperative monitoring for ruptured intracranial aneurysms focuses on preventing secondary brain injury and minimizing complications. Possible complications are divided mainly into two types depending on the onset time, with rebleeding, hydrocephalus, and seizure as early complications within 72 h and delayed cerebral ischemia (DCI) in the delayed phase after 72 h [5].
In this case, two intracranial aneurysms were found on initial imaging, and considering the initial postoperative progress and postoperative images, it was less likely that the initial rupture site failed to be identified. Because it was judged to be a rupture of another intracranial aneurysm rather than a re-rupture of the initial rupture site. However, considering that the location of the ICH observed on brain CT at postoperative day 17 coincided with the location of the rupture of the intracranial aneurysm found on postoperative day 23, it is possible that the patient’s prognosis would have been different if a more aggressive treatment, such as performing cerebral angiography earlier, was considered.
CONCLUSIONS
This patient had two intracranial aneurysm hemorrhages within one hospitalization period, which is unusual. Considering that one of the risk factors for SAH is a history of SAH due to ruptured intracranial aneurysm, patients with multiple intracranial aneurysms need to be observed more closely than those with a single ruptured intracranial aneurysm, even if the patient’s progress is good. It is vital not to focus only on representative cases that may occur depending on the timing of complications after performing embolization but also not to exclude the possibility of rupture of other intracranial aneurysms.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
