|
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(4); 2023 > Article |
|
Abstract
Objective
While patients with medically intractable acute cerebellar infarction typically undergo suboccipital craniectomy and removal of the infarcted tissue, this procedure is associated with long operating times and postoperative complications. This study aimed to investigate the effectiveness of minimally invasive navigation-guided burr hole aspiration surgery for the treatment of acute cerebellar infarction.
Methods
Between January 2015 and December 2021, 14 patients with acute cerebellar infarction, who underwent navigation-guided burr hole aspiration surgery, were enrolled in this study.
Results
The preoperative mean Glasgow Coma Scale (GCS) score was 12.7, and the postoperative mean GCS score was 14.3. The mean infarction volume was 34.3 cc at admission and 23.5 cc immediately following surgery. Seven days after surgery, the mean infarction volume was 15.6 cc. There were no surgery-related complications during the 6-month follow-up period and no evidence of clinical deterioration. The mean operation time from skin incision to catheter insertion was 28 min, with approximately an additional 13 min for extra-ventricular drainage. The mean Glasgow Outcome Scale score after 6 months was 4.8.
Cerebellar infarction accounts for approximately 2-3% of all strokes [2]. According to some studies, approximately 20% patients with cerebellar stroke develop signs of clinical and radiographic deterioration due to mass effect [2,7]. Transtentorial herniation and hydrocephalus, as well as brainstem and fourth ventricle compression, are potentially life-threatening complications resulting from cerebellar space-occupying lesions. This is particularly true given the limited compensation space afforded by the posterior fossa [12].
In patients with malignant cerebellar edema, decompressive suboccipital craniectomy (DSC) and external ventricular drainage (EVD) are currently recommended as the therapy of choice in the guidelines of the American Stroke Association and the European Stroke Organization [4]. However, this procedure is particularly stressful for elderly patients and those receiving anticoagulants owing to long operating times and surgery-related tissue damage. In addition, the large skin incision and durotomy required for the procedure can result in surgery-related complications. The aim of this study was to investigate the clinical usefulness and effectiveness of burr hole aspiration surgery, performed using a navigation system, for cerebellar infarction.
All study protocols were approved by the institutional review board of our institute, and the need for informed consent was waived due to the retrospective nature of this study. A retrospective review of 14 patients who underwent navigation-guided burr hole aspiration surgery for acute cerebellar infarction from January 2015 to December 2021 was conducted. On admission, all the patients were evaluated using the Glasgow Coma Scale (GCS) score. Diagnosis of acute cerebellar infarction was performed based on magnetic resonance imaging (MRI), and computed tomography (CT) images were performed when clinical exacerbation was detected. Various factors including the infarction volume, presence of hydrocephalus, degree of 4th ventricle compression and brain stem compression were evaluated. Patients with neurological deterioration that GCS score was less than 13 were performed burr hole aspiration. And even if GCS score was 13 or 14, infarction volume was greater than 15 ml were performed navigation-guided burr hole aspiration (Fig. 1A, B). If the patient exhibited progressive mental deterioration associated with hydrocephalus caused by fourth ventricle obliteration, additional EVD was performed. The Glasgow Outcome Scale (GOS) used to assess patient outcomes, and complications were evaluated after 6 months (Table 1).
Under general anesthesia, the patients were placed in the prone position on the operating table, and head was fixed by using three-pin Mayfield fixators. Using the navigation system, the catheter insertion site was determined to find the shortest trajectory and longest axis aimed at the infarct center, avoiding the sinus and midline (Fig. 2A, B). A 3-cm skin incision was made perpendicular to the catheter insertion site, and the burr hole was made using a high-speed drill. The 12F catheter was inserted through the burr hole using the navigation system so that it could be positioned along the longest axis possible, and a catheter was inserted to allow the side hole to be located at the infarction site, to the maximum extent possible. Using a 10 cc syringe, careful manual aspiration of the infarcted tissue was performed with slightly negative pressure (Fig. 3). After adequate aspiration, the catheter was retained in the remnant infarcted tissue, and then tunneled subcutaneously and connected with a conventional cerebrospinal fluid (CSF) collection system. If the patients have confirmed to have hydrocephalus, EVD was performed through the occipito-parietal point [13].
The color and volume of the drained infarcted tissue was monitored daily. If the infarcted tissue was effectively drained every day, brain CT was performed 3 days after surgery. If no drainage was found, CT was performed immediately. If the infarcted tissue was not found to have been sufficiently removed on follow-up brain CT, the drainage catheter was retained for two additional days, with periodic squeezing of the drain catheter. Follow-up CT was performed 7 days postoperatively, and the infarction volume was assessed (Fig. 4A, B).
Patient characteristics and surgical results are summarized in Table 2. The patients’ age ranged from 53 to 83 years. The mean infarction volume on brain CT at admission was 34.3 cc (range, 17.1-46.7 cc). The mean preoperative and postoperative GCS scores were 12.7 (range, 10-14) and 14.3 (range, 13-15), respectively. The mean operation time was defined as the time from the skin incision to the insertion of the catheter. In the 9 cases which involved insertion of the catheter into the infarcted tissue only and no EVD, the mean operation time was 28 min. An additional 13 min (on average) were needed in 4 patients for EVD. The average infarction volume evacuated through the catheter during the operation was 11 ml. The mean infarction volume immediately following surgery was 23.5 cc. On postoperative day 7, the residual mean infarction volume was 15.6 cc. In 5 cases, we performed EVD with the placement of a catheter, which was retained for an average of 4 days. The mean follow-up duration was 12 months, and the GOS score calculated in all patients for an average of 6 months following surgery. Eleven of the 14 patients showed good recovery; the remaining 3 patients continued to exhibit moderate disability. The mean GOS score was 4.8. Among the patients with a high GCS score (over 10 points) at admission, 7 showed good recovery and displayed minimal or no brain stem compression. There were no surgery-related complications, including bleeding, infection, or pseudomeningocele formation.
The cerebellum and brain stem are located at the tightly constrained posterior fossa, and are bound above by the tentorium cerebelli and below by the base of the skull. Due to these anatomical features, edema after cerebellar infarction can easily compress the fourth ventricle or aqueduct of sylvius, and interrupt the flow of CSF, ultimately leading to obstructive hydrocephalus. The natural pathological process of this cerebellar infarction itself is very dangerous [15]. Macdonell et al. [10] reported that cerebellar infarcts account for approximately 1.5% of the total cerebral infarcts, but the corresponding mortality rate is approximately 23%, which is significantly higher than that of the other cerebral infarctions. A serious and immediate threat to life due to cerebellar infarction is usually caused by brain stem compression due to cerebellar swelling. In most patients with space-occupying cerebellar infarction, obstructive hydrocephalus causes early signs of clinical deterioration [6]. Raco et al. [13] recommended EVD as the first choice of intervention, and considered DSC only in the event of further clinical deterioration. However, EVD alone may be dangerous in these patients because it not only carries the risk of inducing upward herniation, but also may not effectively relieve brain stem compression, especially in the context of increasing mass effect [4]. Currently, DSC combined with EVD is considered the appropriate strategy. In a recent study, preventive DSC was found to be associated with good clinical outcomes in patients with cerebellar infarction [6]. DSC has the advantage of assuring effective decompression and direct removal of the infarcted tissue. However, it requires long operation times and incurs additional risk due to operation-induced tissue damage. Further, DSC can result in patient instability, additional brain injury, bleeding, pseudomeningocele formation, and wound infection in the postoperative period [3]. Koh et al. [7] reported that infarct volume and the territorial distribution of infarcts do not differ between patients who deteriorate and those who remain stable. They found that only hydrocephalus, brain stem deformity, and basal cistern compression on CT or MRI appear to correlate with clinical deterioration, all of which present challenges to the performance of DSC. Based on an analysis of long-term clinical outcomes after DSC, Jüttler et al. [4] concluded that the value of different treatment strategies for space-occupying cerebellar infarction and prognostic factors for patient selection remain unclear.
As mentioned previously, the tight posterior fossa provides limited space for the compensation of mass effect, and life-threatening brain stem compression may develop rapidly [4]. Therefore, surgery is widely accepted as the treatment of choice in cerebellar stroke with mass effect, and strongly recommended in the current stroke guidelines [9]. Based on our experience with stereotactic burr hole aspiration surgery in patients with cerebellar hemorrhage [9], we applied this treatment method for patients with acute cerebellar infarction. Theoretically, minimally invasive surgery would be optimal provided it can adequately remove the infarcted tissue [8].
The average cerebellar volume for Koreans is 128.6±14.1 ml, which is slightly lower than the volume reported by Raz et al. [14] (136.7±11.7 ml) and MacLullich et al. [11] (144.2±15.4 ml). It is not clear whether this difference is due to racial variation or the different methods of volume measurement. In our study, the mean infarction volume on brain CT at admission was 34.3 ml, and the volume of infarction evacuated through the catheter during the operation was 11-15 ml. As a result, a sufficient decompression effect and clinical improvement could be expected when approximately 30-50% of the infarcted tissue was aspirated. In our institute, previously reported advantages of navigation-guided burr hole aspiration surgery include the short operation time, decreased risk of additional brain injury, small incision size, lower complication rate, and the fact that it can be performed without durotomy [5,9]. In our opinion, this operation is not only suitable for cerebellar hemorrhage but also for cerebellar infarction. Burr hole aspiration is appropriate for patients with mild brain stem compression, inability to undergo a long-duration surgery, or poor medical condition or coagulation disorder. It may also be useful for the patients at risk of fourth ventricle obstruction due to the progression of brain swelling who still exhibit patent CSF flow.
Our study has some limitations. The number of patients enrolled in this study was relatively small. Further studies with a larger number of patients and a scrupulous prospective set of clinical data are necessary for more accurate elucidation of the predictive factors for successful navigation-guided burr hole aspiration. And in our institution, primary evaluation of cerebral infarction is perfomed by the neurologist, when patients has mild neurologic symptom, or no surgery is required. After patients progressed neurologic symptoms, and when surgery is required due to cerebellar edema or hydrocephalus, neurosurgeon evaluates the patients and proceeds the treatment. And most patients have a GCS score of 12 or higher, so further research is needed to determine the optimal timing of navigation guided burr hole drainage [13]. So additional evaluation of early surgery and recovery of the patient’s neurologic symptoms is necessary.
Fig. 1.
A 65-year-old male patient with acute cerebellar infarction. (A) Brain CT at admission shows acute cerebellar infarction with obstruction of the fourth ventricle (arrows) and mild brain stem compression. The infarction volume is 30.9 ml. (B) CT shows third ventricle dilatation (asterisks) and lateral ventricle anterior horn dilatation (arrows). CT, computed tomography
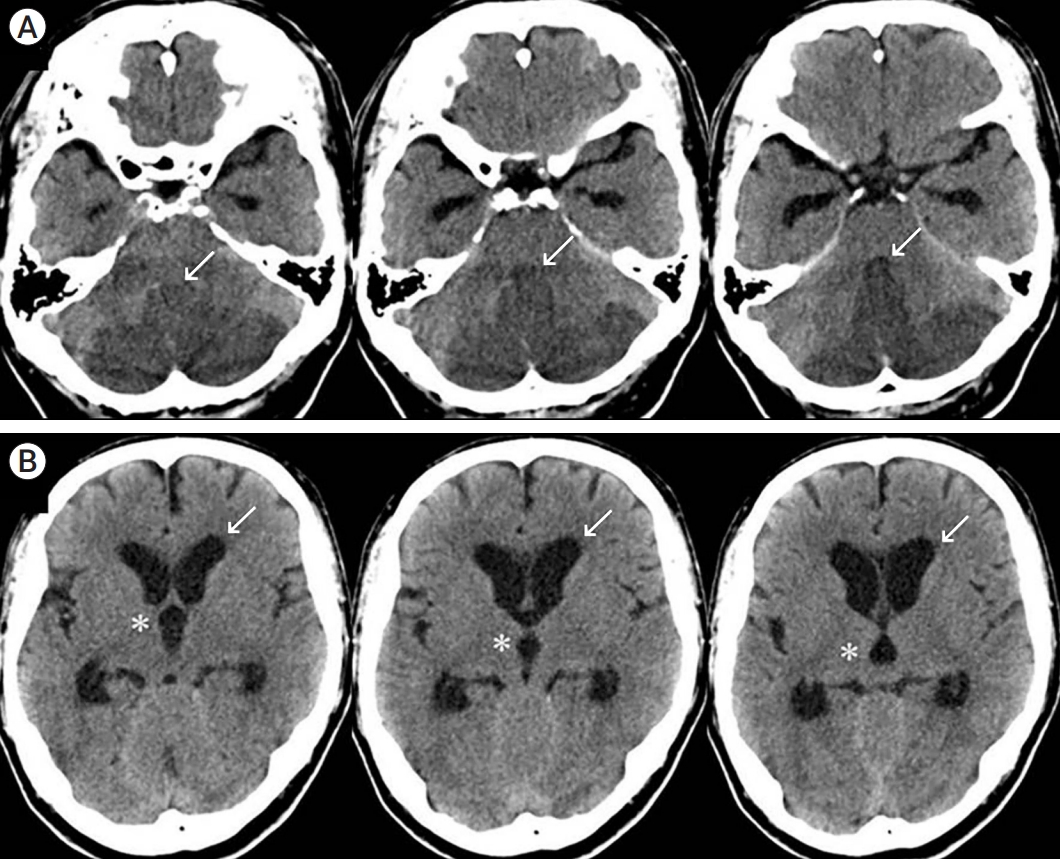
Fig. 2.
(A) Postoperative CT; the asterisk shows catheter insertion to allow the side hole to be located at the infarction site to the maximum extent possible. The arrow shows relaxation of the fourth ventricle compression. (B) CT shows improvement of the third ventricle dilatation (asterisks). The arrow shows reduction of the lateral ventricle anterior horn expansion. CT, computed tomography
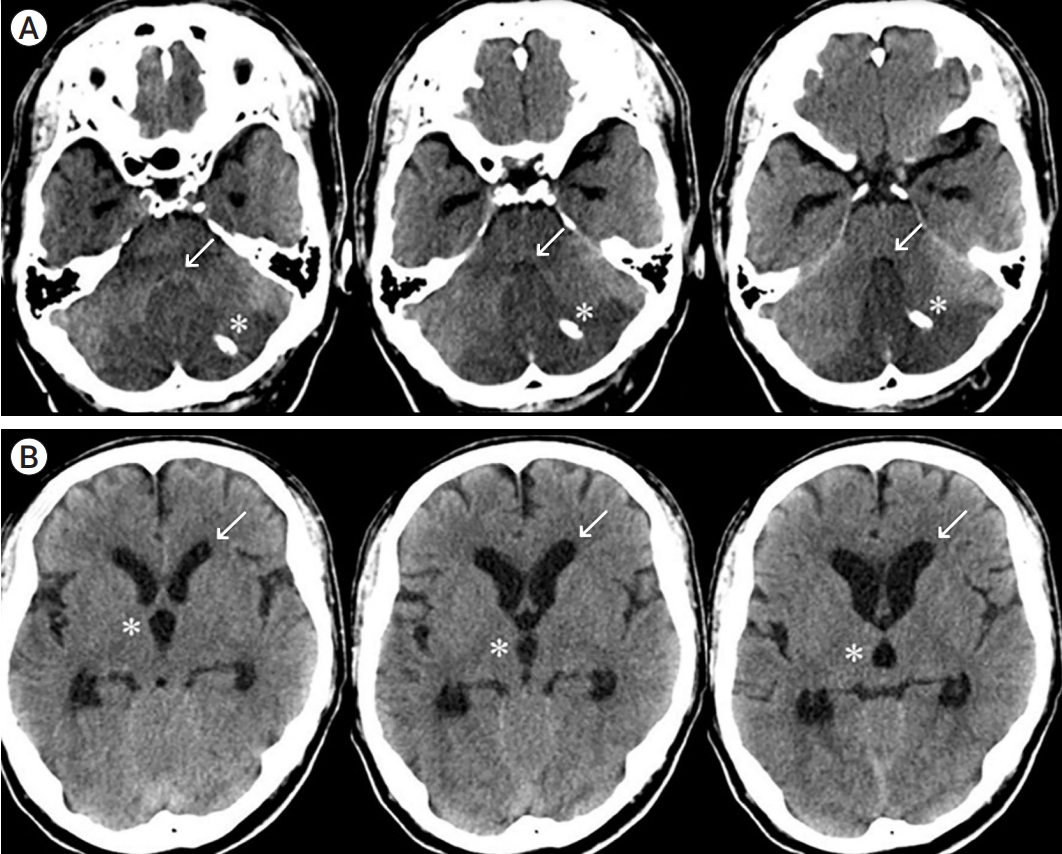
Fig. 3.
Intraoperative photograph shows a 3-cm skin incision and insertion of the catheter through the burr hole. The aspirated infarction tissue is observed in the inserted catheter.
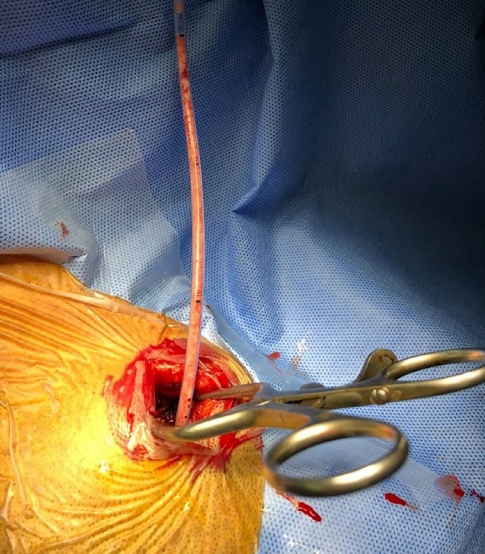
Fig. 4.
After 7 days, CT shows more improvement in the mass effect. (A) The arrow shows a dilated fourth ventricle, and the asterisk shows reduction of the lateral ventricle anterior horn expansion. (B) The asterisk shows favorable changes in the third ventricle dilatation, and the arrow indicates a normal-sized lateral ventricle anterior horn. CT, computed tomography
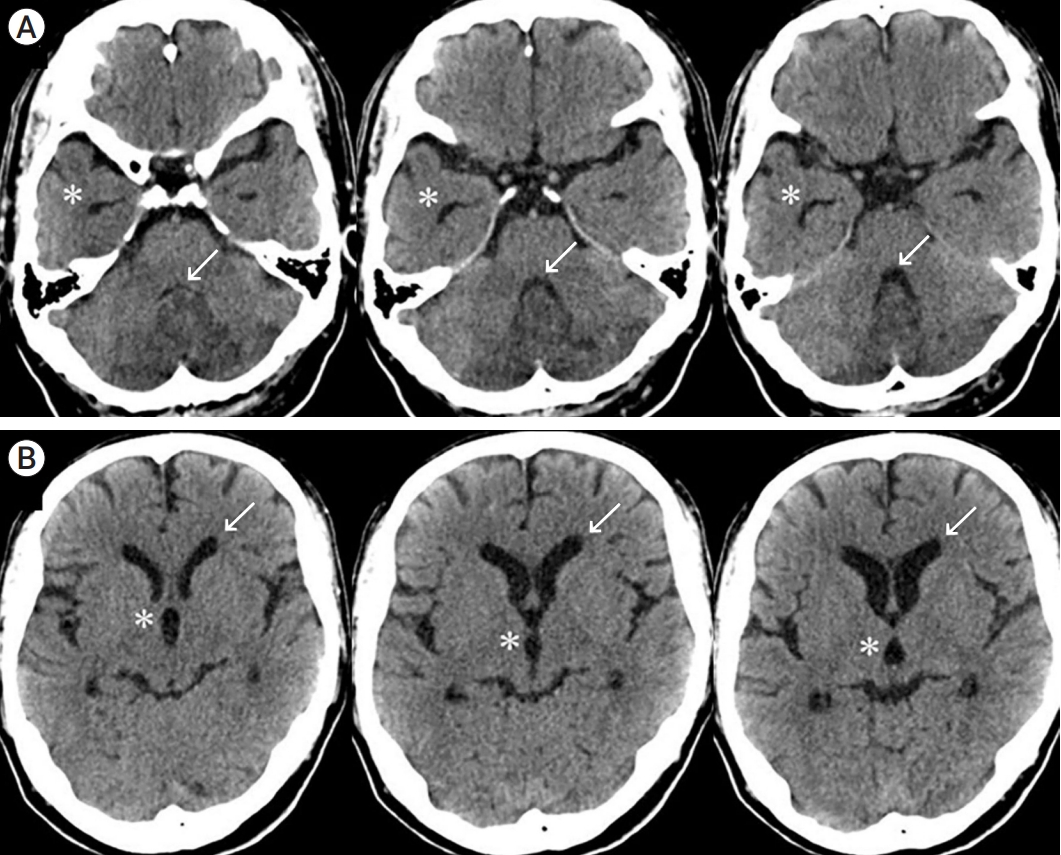
Table 1.
Characteristics of patients with acute cerebellar infarction
Table 2.
Characteristics and surgical results of the patients
| Variables | Value |
|---|---|
| No. of patients | 14 |
| Males/females | 11/3 |
| Mean age (range) | 60 (53-83) |
| Preop. GCS score | |
| Mean (range) | 12.7 (10-14) |
| Postop. GCS score | |
| Mean (range) | 14.3 (13-15) |
| CT finding | |
| Mean preop. infarction volume (range) | 34.3 (17.1-46.7) |
| Mean postop. infarction volume (range) | 23.5 (10.1-34.7) |
| Mean 7 days postop. infarction volume (range) | 15.6 (6.8-23.3) |
| Hydrocephalus | 5 |
| Mean operation time* (min) | 28 |
| EVD | 5 |
| Mean follow-up duration (months) | 12 |
| GOS score after 6 months | |
| Good recovery | 11 |
| Moderate disability | 3 |
| Severe disability | 0 |
| Vegetative state | 0 |
| Dead | 0 |
REFERENCES
1. Amar AP. Controversies in the neurosurgical management of cerebellar hemorrhage and infarction. Neurosurg Focus. 2012 Apr;32(4):e1.

3. Hadanny A, Rozovski U, Nossek E, Shapira Y, Strauss I, Kanner AA, et al. Craniectomy versus craniotomy for posterior fossa metastases: Complication profile. World Neurosurg. 2016 May;89:193-8.


4. Jüttler E, Schweickert S, Ringleb PA, Huttner HB, Kohrmann M, Aschoff A. Long-term outcome after surgical treatment for space-occupying cerebellar infarction: Experience in 56 patients. Stroke. 2009 Sep;40(9):3060-6.


5. Khoshnevisan A, Allahabadi NS. Neuronavigation: Principles, clinical applications and potential pitfalls. Iran J Psychiatry. 2012 Spring;7(2):97-103.


6. Kim MJ, Park SK, Song J, Oh SY, Lim YC, Sim SY, et al. Preventive suboccipital decompressive craniectomy for cerebellar infarction: A retrospective-matched case-control study. Stroke. 2016 Oct;47(10):2565-73.


7. Koh MG, Phan TG, Atkinson JL, Wijdicks EF. Neuroimaging in deteriorating patients with cerebellar infarcts and mass effect. Stroke. 2000 Sep;31(9):2062-7.


8. Kwon WK, Park DH, Park KJ, Kang SH, Lee JH, Cho TH, et al. Prognostic factors of clinical outcome after neuronavigation-assisted hematoma drainage in patients with spontaneous intracerebral hemorrhage. Clin Neurol Neurosurg. 2014 Aug;123:83-9.


9. Lee JH, Kim DW, Kang SD. Stereotactic burr hole aspiration surgery for spontaneous hypertensive cerebellar hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2012 Sep;14(3):170-4.



10. Macdonell RA, Kalnins RM, Donnan GA. Cerebellar infarction: Natural history, prognosis, and pathology. Stroke. 1987 Sep;18(5):849-55.


11. MacLullich AM, Edmond CL, Ferguson KJ, Wardlaw JM, Starr JM, Seckl JR, et al. Size of the neocerebellar vermis is associated with cognition in healthy elderly men. Brain Cogn. 2004 Dec;56(3):344-8.


12. Neugebauer H, Witsch J, Zweckberger K, Juttler E. Spaceoccupying cerebellar infarction: complications, treatment, and outcome. Neurosurg Focus. 2013 May;34(5):e8.

13. Raco A, Caroli E, Isidori A, Vangelista T, Salvati M. Management of acute cerebellar infarction: One institution’s experience. Neurosurgery. 2003 Nov;53(5):1061-5; discussion 1065-6.



- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,303 View
- 39 Download
- ORCID iDs
-
Dae-Won Kim

https://orcid.org/0000-0003-2151-2841 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print