Location and Characteristics of Warfarin Associated Intracranial Hemorrhage
Article information
Abstract
Objective
In the so-called primary intracerebral hemorrhage (ICH), lobar and deep ICH were mainly due to cerebral amyloid angiopathy and deep perforating arterial disease. Our aim was to identify specifics of warfarin associated ICH (WAICH) and to focus on differences in susceptibility to warfarin according to the underlying vasculopathies, expressed by ICH location.
Materials and Methods
We identified all subjects aged ≥ 18 years who were admitted with primary ICH between January 1, 2007 and September 30, 2012. We retrospectively collected demographic characteristics, the presence of vascular risk factors and pre-ICH medication by chart reviews. We categorized ICH into four types according to location: lobar, deep, posterior fossa, and undetermined. We investigated characteristics (including hematoma volume and expansion) of ICH according to the location of ICH.
Results
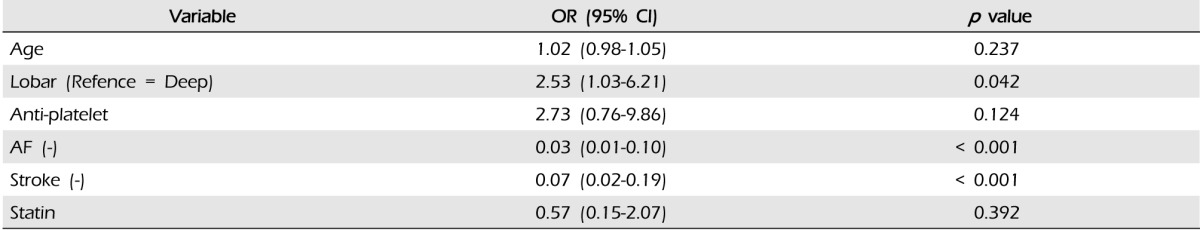
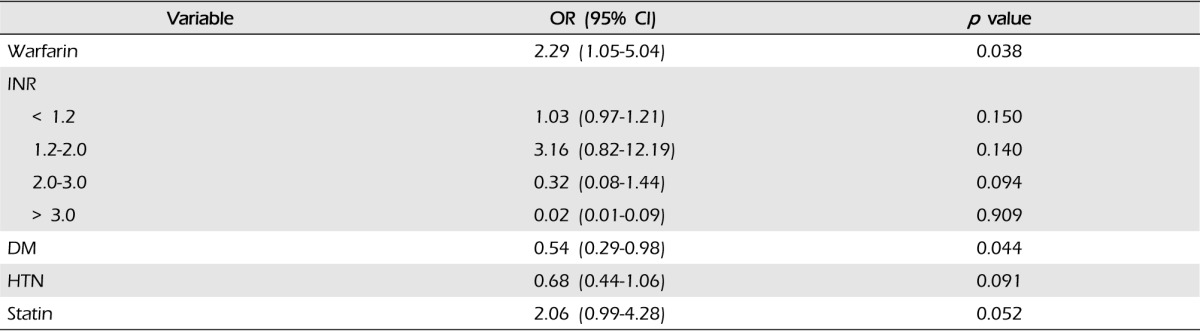
WAICH accounted for 35 patients (5.6%) of 622 ICH cases. In WAICH, 13 patients (37.1%) had lobar ICH and 22 patients (60.0%) had non-lobar ICH. Compared to other locations of ICH, lobar ICH showed an excess risk of WAICH (OR 2.53, 95% CI 1.03-6.21, p = 0.042). The predictors of lobar location of ICH were warfarin (OR 2.29, 95% CI 1.05-5.04, p = 0.038) and diabetes mellitus (DM) (OR 0.54, 95% CI 0.29-0.98, p = 0.044). The lobar location of ICH showed significant association with larger hematoma volume (p = 0.001) and high ratio of hematoma expansion (p = 0.037) compared with other locations of ICH.
Conclusion
In our study, warfarin showed significant association with lobar ICH and it caused larger hematoma volume and more expansion of hematoma in lobar ICH.
INTRODUCTION
The high prevalence of atrial fibrillation in the aging population has led to an increasing incidence of warfarin associated intracerebral hemorrhage (WAICH).10)21) Although WAICH is predictive of larger hematoma volume and high rates of hematoma expansion and worse clinical outcome than primary ICH, the mechanisms of these results were not completely understood.5)11)
Many diseases may lead to ICH; two prominent causes of primary ICH are cerebral amyloid angiopathy in lobar ICH and deep perforating artery vasculopathy in deep ICH.2)20) This was indicated with autopsy in prognosis of an ICH (PITCH) cohort.7) The impact of warfarin on these types of ICH was distinct, which suggests different sensibilities of these two vasculopathies on warfarin. To date, only Nelly's article reported on the impact of warfarin according to the underlying vasculopathies.7) This article reported an association of vitamin K antagonists use with larger hematoma volume only in non-lobar ICH.7)
We aimed at identification of specifies of WAICH and focused on differences in susceptibility to warfarin according to the underlying vasculopathies, expressed by the ICH location.
MATERIALS AND METHODS
We identified all subjects aged ≥ 18 years who were admitted with primary ICH to Busan-Ulsan Regional Cardiocerebrovascular Center between January 1, 2007 and September 30, 2012. Exclusion criteria were traumatic ICH, hemorrhagic transformation of cerebral infarction, ICH secondary to vascular malformation, tumor, aneurysm, and vasculitis of the central nervous system. In addition, we excluded patients with primary intraventricular hemorrhage (IVH), liver cirrhosis, and other coagulopathies.
Demographic characteristics and medical history
We retrospectively collected demographic characteristics, medical history of vascular risk factors such as hypertension (HTN), diabetes mellitus (DM), smoking, hyperlipidemia, previous stroke, atrial fibrillation (AF), ischemic heart disease, congestive heart failure (CHF), heart valve disease, pulmonary thromboembolism (PTE), liver disease, chronic kidney disease (CKD), and pre-ICH medication by chart review.
Clinical and laboratory study at admission
We assessed the severity of neurological deficit by Glasgow coma scale (GCS) and measured the international normalized ratio (INR) value at admission. For WAICH patients, the INR values obtained before reversals were used for this report. WAICH patients were stratified by level of anticoagulation (INR < 1.2, 1.2-2.0, 2.0-3.0, > 3.0).
Radiological analysis
We checked computed tomography (CT) scans at admission and after 4 hours in all ICH patients, with continuous slices, no gap, and 5 mm slice thickness. We distributed the location of ICH: (1) lobar (frontal, temporal, parietal, and occipital) when the origin of hemorrhage appeared to be in the cerebral hemisphere superficial to the deep gray matter structure; (2) deep when originating from basal ganglia and thalamus (3) in the posterior fossa when originating from the brain stem or cerebellum and (4) undetermined in case of ICH when the origin could not be identified and including huge ICH cases that overlapped two categories. We calculated the volume of ICH according to the ABC/2 method.19) The expansion of hematoma was defined as increasing by 33% and 6 cc in hematoma volume over 4 hours.
Statistical analyses
To ascertain factors associated with warfarin, we used χ2-test and independent samples t-test for comparison of average values in all ICH patients. We explored the influence of warfarin on volume and hematoma expansion, reflecting the influence of the ICH location (Lobar vs Deep). Therefore, intending lobar and deep ICH, we performed multiple linear regression analysis including the location of ICH, taking warfarin, and others. After dividing these ICH patients according to the location of ICH, we repeated multiple linear regression analysis. In lobar and deep ICH, multiple logistic regression analysis was performed to estimate the independent contributions of variables. Statistical analysis was performed using SPSS version 12.0 (SPSS Inc., Chicago, IL, United States). All p values less than 0.05 were considered statistically significant.
RESULTS
Study population and prevalence of warfarin
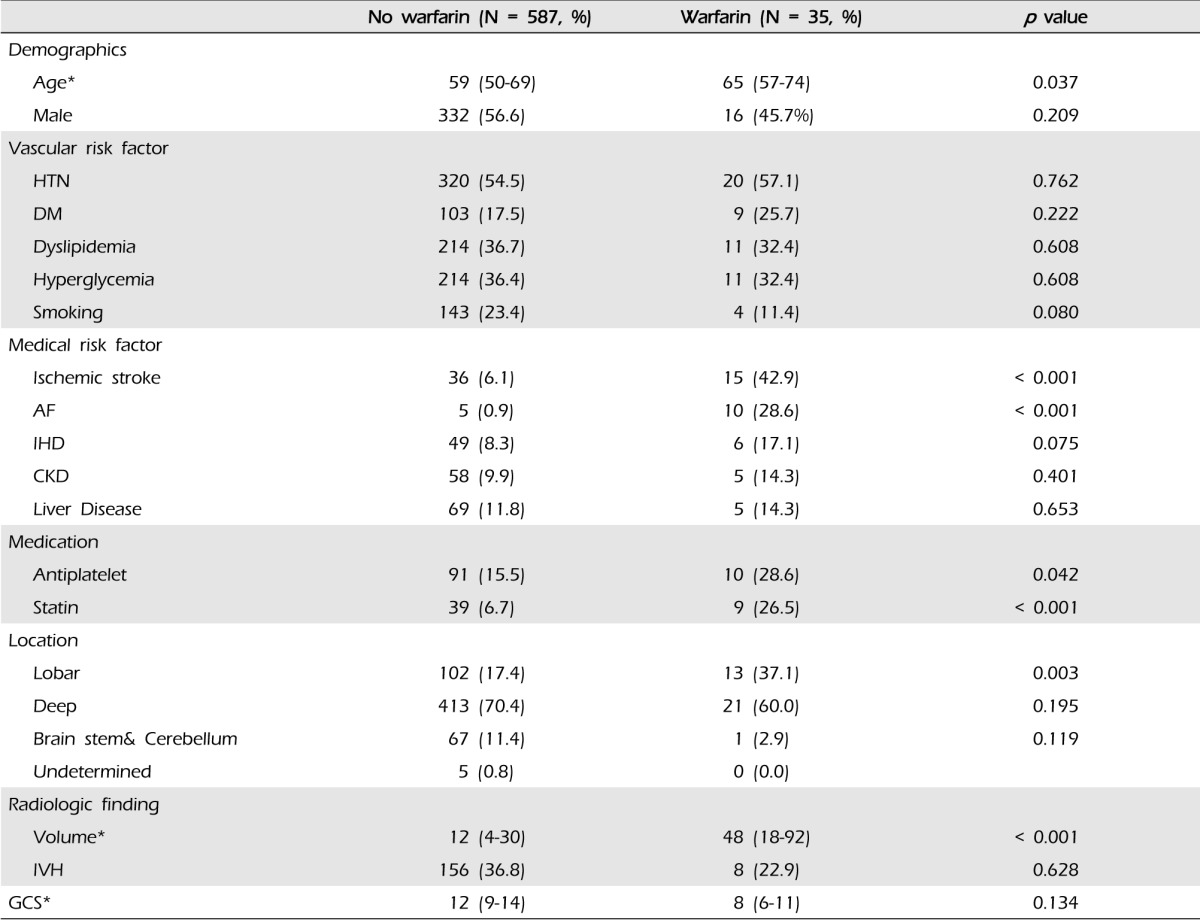
We included 622 patients (Median age, 52; interquartile range [IQR], 33-76), 348 were men (55.9%). Thirty five patients (5.6%) were taking warfarin at admission. Fourteen patients (40%) took warfarin for heart valve diseases, seven (20%) for AF, seven (20%) for ischemic stroke, four (11%) for PTE, and three (9%) for venous thromboembolism. The characteristics of patients taking warfarin versus those not taking warfarin are shown in Table 1. In univariate analysis, factors associated with WAICH patients were older age, comorbidities such as AF, history of previous ischemic stroke and pre-ICH medication (antiplatelet and statin). Patients taking warfarin had a more severe neurologic defect at admission: median GCS 12 (9-14) in the not taking warfarin group versus eight in WAICH (6-11), however, there was no statistically significant difference (p = 0.134).
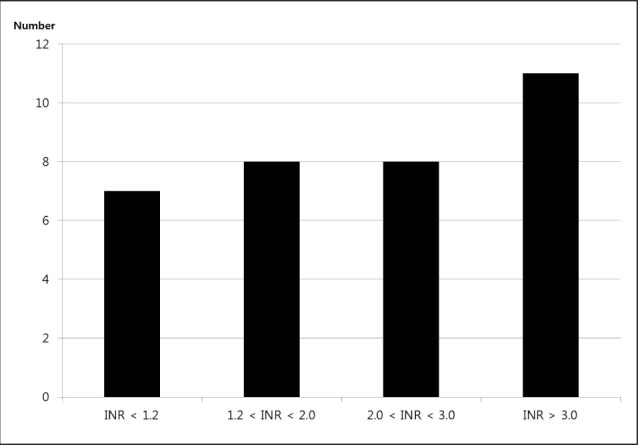
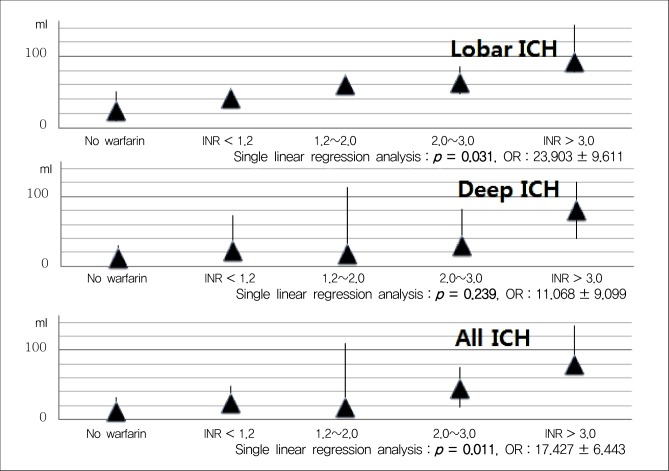
At admission, the median INR value was 2.1 (IQR 1.2-3.3) in all WAICH patients, 2.9 in lobar (IQR, 2.1-3.6), 1.7 in deep (IQR, 1.0-2.9), and 0.9 in posterior fossa (p = 0.202). The numbers of WAICH patients categorized according to the INR value and are shown in Fig. 1. Among patients reported to be taking warfarin, only eight (23.8%) patients had therapeutic INR (2.0-3.0). The categories of INR value classified according to the location of ICH are shown in Table 2. The number of lobar ICH patients in WAICH whose INR value under 2.0 was three (20%), while that of the lobar patients whose INR value over 2.0 was 10 (52.6%) (p = 0.051).
Effects of warfarin on ICH volume
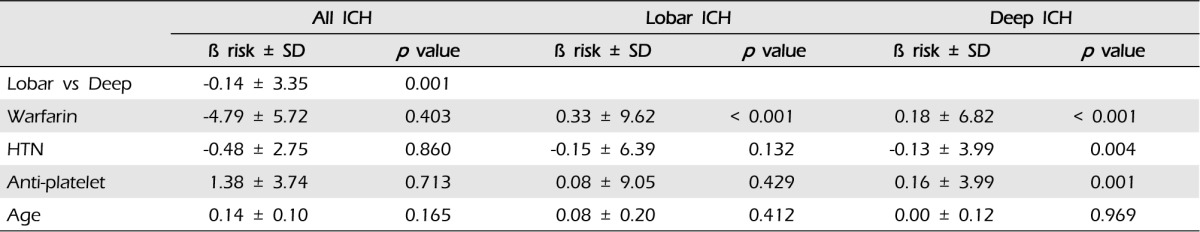
Taking warfarin showed significant association with a larger volume of ICH compared with the other group (p < 0.001) (Fig. 2). In multiple linear regression on all documented patients, the only predictor of larger hematoma was the location of ICH (p = 0.001). After sorting our cohorts according to the location of ICH, we performed multiple linear regression analysis (Table 3) ; both in lobar ICH and in deep ICH, patients taking warfarin showed larger hematoma volume (p < 0.001, p < 0.001). To elucidate a dose-response relationship between INR value and volume of ICH, we performed an analysis with INR categories. We found that patients with higher INR value expressed larger ICH in all ICH patients (p = 0.011); in lobar ICH, INR values showed an association with the volume (p = 0.031), but in deep ICH, the volume was not influenced by INR values (p = 0.239) (Fig. 3).
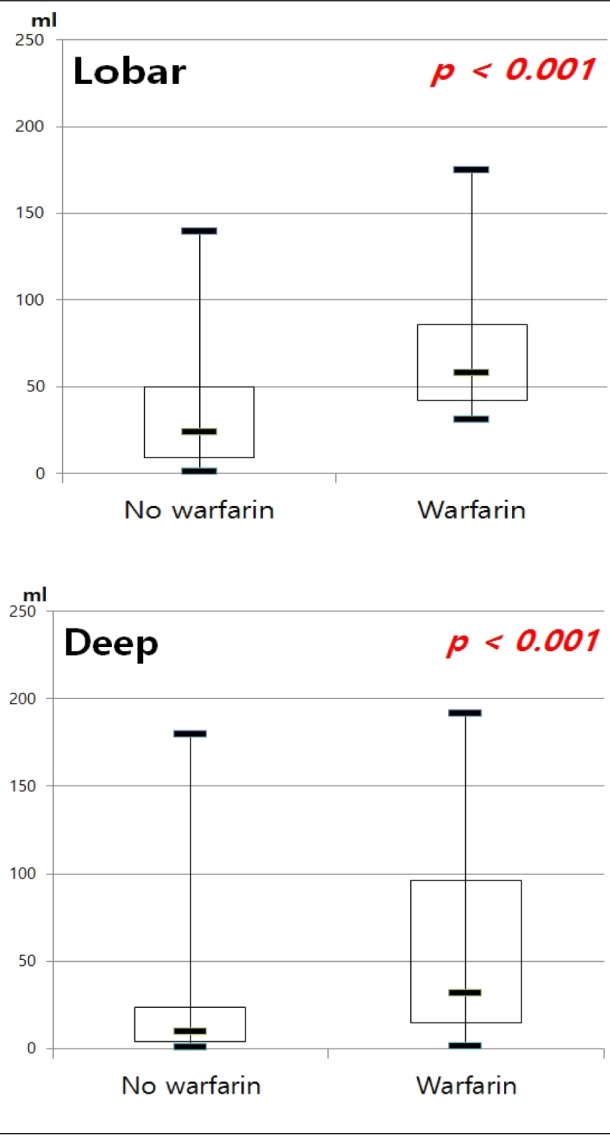
Median intracerebral hemorrhage volume between patients with and without warfarin. ICH = intracerebral hemorrhage
Effect of warfarin on hematoma expansion
In our cohorts, more than 33% of hematoma volume was increased in 144 cases and hematoma volume of 6 cc or more was increased in 92 cases. In our cohorts, 44 cases showed a hematoma increase of both percentage and volume.
Taking warfarin showed significant association with the expansion of ICH (p < 0.001). In our cohorts, the only predictor of expansion was the lobar location of ICH (p = 0.037, OR 2.08, 95% CI:1.05-4.14). After dividing patients according to the location of ICH, we performed multiple linear regression analysis (Table 4): (1) in lobar ICH, patients under warfarin showed more hematoma expansion (p = 0.001); and (2) in non-lobar ICH patients, taking warfarin also influenced the expansion of ICH (p < 0.001) (Fig. 4).
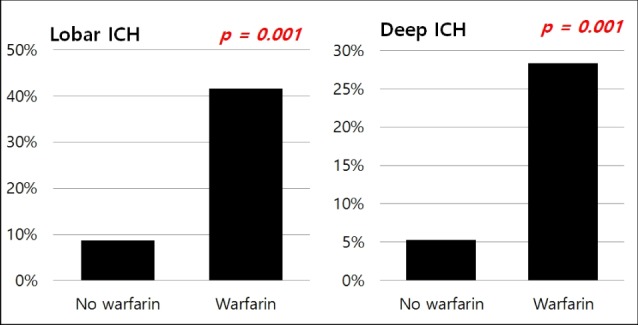
Ratio of hematoma expansion between patients with and without warfarin. ICH = intracerebral hemorrhage
To elucidate a dose-response relationship between INR value and the ratio of expansion in ICH, we performed an analysis with INR categories. We found that higher INR value was not associated with the expansion of hematoma in all ICH patients (p = 0.145). After dividing the location of ICH, expansion of hematoma was not affected by INR value (Fig. 5).
Probability of WAICH and lobar hemorrhage
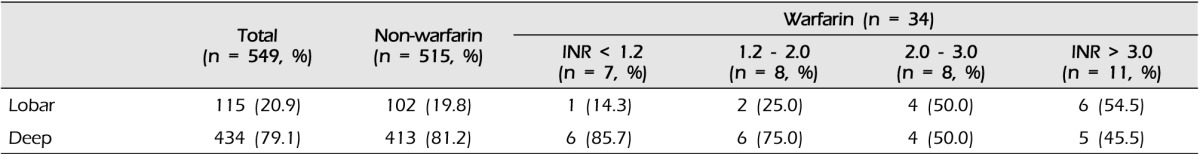
Results of multivariate logistic regression analysis of the risk factors in WAICH are shown in Table 5. History of AF and ischemic stroke showed strong association with WAICH. Compared with other ICH locations, only lobar location showed an excess risk of WAICH (OR 2.53, 95% CI 1.03-6.21). The history of antiplatelet medication showed the highest odds ratio in our cohort, but did not reach statistical significance. (p = 0.124, OR 2.73, 95% CI 0.76-9.86) The results of multivariate analysis of lobar ICH are shown in Table 6. A history of DM and taking warfarin were significant risk factors of lobar ICH. Although taking warfarin was the most important risk factor of lobar ICH, intensity of anticoagulation did not affect the lobar location of ICH.
DISCUSSION
Approximately 1% of the population is currently taking warfarin and this ratio has shown an increase in recent studies.14) Previous studies reported that 5~12% of ICH occurred by oral anticoagulation.6)14)18) Patients with WAICH had worse outcome.7)11) In our study, we have found the relationship between WAICH and underlying vasculopathies such as amyloid angiopathy and deep perforating arterial disease, which may contribute to the location of ICHs.
In previous reports, advanced age, HTN, history of cerebrovascular disease, and intensity of anticoagulation (mainly if INR > 4.0) were predisposing factors of WAICHs.1)14) Consistent with previous reports, we found that WAICH patients were older and had more vascular risk factors compared with the other group. Although value of INR more than 4.0 was a significant risk factor of WAICH, most WAICH occurred at INR < 3.0.9)17) In our study, 60% of our patients were within or below the therapeutic INR range and median INR value was 2.2 (IQR 1.2-3.3). In patients taking warfarin, as well as in the normal range INR value, physicians should pay attention.
In two previous reports, larger baseline volume of hematoma in WAICH was the major difference as compared to primary ICH.4)23) We also found that patients using warfarin had larger hematoma volume. The relationship between anticoagulation intensity measured on admission and hematoma volume was controversial. Flaherty's study reported that only INR > 3.0 showed an association with larger hematoma volume, however, another study published by Berwaert reported that larger hematomas did not show correlation with higher INR value.3)11) In our study, significant association was observed between the anticoagulation intensity measured on admission and the hematoma volume, like other reports.15)
In patients taking warfarin, we found that the hematoma volume differed depending on the location of ICH. In our study, the lobar location was the most important factor affecting the volume of ICH and the intensity of anticoagulation was relative to the volume of ICH only in lobar ICH. However, Nelly's cohort study, the only paper reporting on the effects of vitamin K antagonist (VKA) on the volume according to location, showed opposites results; deep ICH, caused by vasculopathy of deep perforating arteries, which seems to lead to larger ICH in VKAs use.7) On the other hand, Rosand's study reported that cerebral amyloid angiopathy was very sensitive to antithrombotics; he identified lobar hemorrhage as the predictor of larger baseline volume.25) Because our report and other studies were limited by inclusion of a small number patients and retrospective reports, prospective extensive research was required in order to confirm a significant association between lobar ICH and larger hematoma volume.
In previous studies, patients taking warfarin at the time of their ICH were associated with increased risk for hematoma expansion.5) History of HTN, hyperglycemia, the lobar location of ICH, and chronic kidney disease were found to show association with greater ICH expansion in some studies.12)27) The INTERACT1 study reaffirmed the importance of hematoma expansion as a dominant contributor to death and dependency in ICH.6) Every 1 ml of hematoma expansion was estimated to be associated with a 5% increase in odds of death and dependency.6) In our study, the intensity of warfarin was not associated with the expansion of ICH. We also found that the location of ICH was the most important factor in hematoma expansion. After dividing patients according to the location of ICH, warfarin was a strong risk factor for hematoma expansion. These results supported that the lobar ICH in patients taking warfarin was expected to have a greater possibility of hematoma expansion.
Results of studies on the location of WAICH have been controversial. Some reports showed a higher proportion of lobar and thalamic hemorrhage in patients taking warfarin.16)24) On the other hand, another study found that taking warfarin was highly associated with the location of cerebellum, particularly in patients with an INR > 2.5.26) In our study, the lobar location of ICH was a significant factor of WAICH and the most important risk factor of lobar hemorrhage was also taking warfarin.
Our study has many limitations. This study had a retrospective design, including the small numbers of WAICH. Time from neurologic symptom onset to initial CT scan may affect the volumes of ICH, however, we did not consider this in our cohort study. In addition, our report used the ABC/2 method for calculation of hematoma volume. This method has been reported to significantly overestimate volume, especially when the shape of hematoma was irregular, which was frequent in WAICH. Another limitation in our study was that the median INR values in each location of WAICH were different. Nevertheless, of the limited size of sample and the different baseline INR, our results showed a strong association between warfarin and lobar ICH, resulting in the larger incidence, initial volume, and expansion of hematoma. Conduct of a prospective larger scale study is necessary in order to understand the exact characteristics of WAICH according to the underlying vasculopathy.
CONCLUSION
In our study, taking warfarin showed a signification association with the occurrence, volume of hematoma, and expansion of lobar ICH. In addition, the intensity of anticoagulation is associated with larger hematoma volume in lobar ICH. In patients suspected as having high risk factor for lobar ICH, such as amyloid angiopathy, attention should be paid to those taking warfarin.
Notes
This work was supported by the Dong-A University Research Fund.