|
 |
| J Cerebrovasc Endovasc Neurosurg > Epub ahead of print |
Abstract
Objective
To assess whether local anesthetic infiltration could minimize the carotid baroreceptor reflex (CBR) which has an incidence after carotid artery stenting (CAS) that varies from 29% to 51%.
Methods
This retrospective single-center study included 51 patients (mean age, 70.47 years) who underwent CAS for carotid stenosis. The groups included patients who underwent CAS for asymptomatic ischemic stroke (n=41) or symptomatic disease (n=10). Preprocedural percutaneous lidocaine injections (PPLIs) were administered to 70.6% and 5.9% of patients who underwent elective CAS and emergency CAS, respectively.
Results
Among patients who received PPLIs, the mean degree of stenosis was 80.5% (95% confidence interval [CI]: ┬▒10.74, 51-98%). The mean distance from the common carotid artery bifurcation to the most stenotic lesion (CSD) was 8.3 mm (95% CI: ┬▒0.97, 6.3-10.2 mm); the mean angle between the internal carotid artery and common carotid artery (CCA) trunk (IAG) was 65.6┬░ (95% CI: ┬▒2.39, 61-70┬░). Among patients who did not receive PPLIs, the mean degree of stenosis was 84.0% (95% CI: ┬▒8.96, 70-99%). The mean CSD was 5.9 mm (95% CI: ┬▒1.83, 1.9-9.9 mm); the mean IAG was 60.4┬░ (95% CI: ┬▒4.41, 51-70┬░). The procedure time was longer in the PPLI group than in the no PPLI group (28.19 [n=39] vs. 18.88 [n=12] days) (P=0.057); the length of intensive care unit stay was shorter in the PPLI group (20.01 [n=36] vs. 28.10 [n=5] days) (P=0.132).
Conclusions
Targeted PPLI administration to the carotid bulb decreased aberrant heart rates and blood pressure changes induced by carotid stent deployment and balloon inflation. As CBR sensitivity increases with decreasing distance to the stenotic lesion from the CCA bifurcation, PPLIs may help stabilize patients during procedures for stenotic lesions closer to the CCA.
Ischemic stroke is the most common cerebrovascular accident, with atherosclerotic carotid stenosis accounting for 15-20% of all cases [26].
Carotid endarterectomy (CEA) has been the treatment of choice for carotid stenosis for more than 60 years [10]; however, carotid artery stenting (CAS) has advanced over the past 30 years and has been recognized as a valid alternative therapeutic option because as it is less invasive and is associated with fewer surgical complications compared with CEA [8].
Atherosclerotic carotid stenosis commonly occurs in the carotid bulb or common carotid artery (CCA) bifurcation [14,27], and the nerve endings of carotid baroreceptors are present in this area of the carotid arterial wall [14,16]. Mechanical dilation of the carotid bulb by stent deployment or balloon inflation can induce serial neuronal responses, such as bradycardia and hypotension, and may lead to perioperative cardiopulmonary and neurological side effects [13].
Hemodynamic instability occurring during CAS has been reported to range from 29% to 51% [14]. Additionally, in one study, differences were observed depending on the location of the stenosis, with carotid baroreceptor reflex (CBR) occurring in 30% of apical lesions, as opposed to 70% of body lesions [30]. The standard management of hemodynamic depression during CAS remains controversial. Although intravenous atropine infusion has been proposed, its indications remain debatable. Some physicians have reported that immediate atropine administration effectively reversed CAS-induced bradycardia in selected patients [20] while others have suggested prophylactic atropine administration for all patients undergoing CAS [7,17,24].
However, prophylactic atropine administration in these patients reportedly increases the risk of paradoxical postprocedural bradycardia [28] and other adverse reactions to atropine, including tachycardia, confusion, urinary retention, and arrhythmia [19,29]. In particular, atropine has been linked to adverse cardiac events in geriatric patients with coronary artery disease [20].
CBR response is a complication of both CAS and CEA; therefore, many surgeons have attempted to address this issue. One such attempt involved direct infiltration of the carotid bulb with lidocaine before excision of the atheroma during CEA. Although this procedure is not consistently effective [2,21], some researchers have attempted to substantiate the effectiveness of intraoperative infiltration of local anesthetic into the carotid bulb during CEA [3].
The aim of this study was to test the hypothesis positing that local anesthetic infiltration can effectively minimize the CBR during CAS.
We conducted a blinded, retrospective study on 51 patients who underwent CAS for carotid stenosis in Jeju Island, Korea from 2017-2022. We retrospectively collected information for each patient, including demographic data such as age, sex, medical condition, or vascular risk factors and several clinical and procedural variables, including the location of the carotid artery and degree of stenosis (%).
This study was approved by the Ethics Committee of Institutional Review Board (approval number: 2023-L11-01), which waived the requirement for informed consent due to the retrospective nature of the study.
The patients were divided into two groups: (I) those who underwent CAS for asymptomatic ischemic stroke (n=41) and (II) those who underwent emergency CAS for symptomatic disease (n=10). Preprocedural percutaneous lidocaine injections (PPLIs) were administered to 70.6% of the patients who underwent elective CAS and 5.9% of the patients who underwent emergency CAS (Fig. 1).
All the endovascular treatments were performed under local anesthesia. For elective cases, preoperative assessments were performed, and dual antiplatelet agents (aspirin 100 mg, clopidogrel 75 mg) were administered for at least 7 days prior to the procedure to prevent ischemic complications. However, for emergency cases, administering antiplatelet agents for 7 days prior to CAS was impossible; therefore, patients were administered a loading dose of antiplatelet agents (aspirin 200 mg, clopidogrel 300 mg) before the procedure, as well as heparin (3,000 units), to prevent intra-procedural ischemic complications [7,23,26,32].
Carotid artery stenosis was estimated using computed tomography angiography or magnetic resonance angiography, and the exact degree of stenosis was confirmed using transfemoral cerebral angiography. The degree of stenosis was assessed using the North American Symptomatic Carotid Endarterectomy trial (NASCET) criteria [11,25].
All procedures were performed according to a standardized CAS protocol [1,4,26] using an embolic protection device (EPD) (Filter Wire EZ Embolic Protection System, Boston Scientific, Marlborough, MA, USA) and a self-expandable stent (Protege RX Tapered Carotid Stent System; ev3, Inc., Plymouth, MN, USA or the Carotid WALLSTENT closed cell self-expanding stent, Boston Scientific). Prior or subsequent dilatation using a balloon was also performed when necessary (Gateway PTA Balloon Catheter, Boston Scientific). After confirming that the carotid artery stenosis had adequately improved, the protective filter was removed. The procedure was concluded after confirming the patency of the stented carotid artery and absence of anomalies in the intracranial vasculature.
A PPLI was administered prior to the CAS procedure. First, a guiding catheter (8Fr guider softip XF, Boston Scientific or 8Fr FlowGate Balloon Guide Catheter, Stryker Neurovascular, Fremont, CA, USA) was introduced into the CCA, and images of the radiopaque-marked carotid bifurcation, including the carotid bulb, were obtained via a road-mapping angiogram (Fig. 2). Subsequently, a needle was introduced transdermally in the neck area under the guidance of real-time fluoroscopy, and 1% lidocaine (4-5 cc) was infiltrated. Before the injection, aspiration was performed to check for any regurgitation of blood to avoid injection of lidocaine into the bloodstream. After infiltrating the carotid apex and bulb with lidocaine, we conversated with the patient to check the hoarseness of their voice and determine the degree of infiltration. The CAS procedure was then performed.
During the procedure, the patientŌĆÖs echocardiogram, arterial blood pressure, pulse, and oxygen saturation were continuously monitored, and after the procedure, patients were closely monitored in the intensive care unit (ICU) until their vital signs stabilized.
The CBR response was computed based on the patientŌĆÖs vital signs, such as heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) before and after carotid stent deployment or balloon inflation, and the largest differences were used in the analysis. The duration of the CAS procedure was measured from the point at which the EPD was introduced to the point at which it was removed. During this period, changes in the patientŌĆÖs vital signs after carotid stent deployment or ballooning were computed and compared with the baseline values (╬öHR, ╬öSBP, ╬öDBP). The greatest changes were defined as the carotid reflex for each patient.
To assess the effect of the CBR response and that of the PPLI, normally distributed (confirmed by Kolmogorov-Smirnov and Shapiro-Wilk tests) continuous variables (╬öHR, ╬öSBP, ╬öDBP) were examined, and the means between the PPLI and no PPLI groups were compared using independent t-tests. Further, continuous variables that were not normally distributed (procedure time, length of ICU stay) were compared between the PPLI and no PPLI groups using the Mann-Whitney U test. The effects of the PPLI on the CBR response and other non-parametric variables were analyzed using these methods. However, given that other variables may have influenced the length of ICU stay in symptomatic patients undergoing emergency CAS, such as the patientŌĆÖs general condition and mental status, statistical analyses were performed only for elective, asymptomatic cases.
To identify other independent variables that could affect the CBR response, we performed simple linear regression and multiple regression analyses using the following independent variables: age, degree of stenosis, distance from the CCA bifurcation to the most stenotic lesion (CSD), mean angle between the internal carotid artery (ICA) and the CCA trunk (IAG), number of balloon inflations, and number of stents deployed. The associations between the CBR response and the other independent variables were examined using Spearman and Pearson correlation analyses. The CSD was defined as the distance from the CCA trunk to the most stenotic point on the ICA, and we used the shortest distance from the CCA bifurcation apex, from which the external carotid artery (ECA) and ICA branch out to the most stenotic area on the stenotic segment (Fig. 3). The IAG was defined as the angle at which the ICA branched off from the CCA trunk. We measured the angle with reference to the midpoint of the horizontal extension from the apex of the CCA bifurcation and the midpoint of the perpendicular extension and from the two-dimensional anterior and posterior margins of the ICA (Fig. 4).
All P values were calculated using two-tailed tests. A P value of < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS software, version 19.0 (IBM Corp., Armonk, NY, USA).
Fifty-one patients who underwent CAS (39 who received a PPLI, 12 who did not receive a PPLI) were included in the final analyses. The mean age of the patients was 70.47 (range: 67.3-73.6) years. Forty-one patients (male:female ratio=3.1:1, 80.4%) underwent elective CAS for asymptomatic ischemic stroke and 10 (male:female=7:3, 19.6%) underwent emergency CAS for symptomatic disease.
Of the 51 patients who underwent CAS, 39 (male:female=3.3:1, 76.5%) received a PPLI, whereas 12 (male:female=1.4:1, 23.5%) did not. Of the patients who received a PPLI, the right:left ratio of the stenotic lesions was 1.1:1, and the mean degree of stenosis was 80.5%. The mean CSD was 8.3 mm, and the mean IAG was 65.6┬░.
Of the patients who did not receive a PPLI, the right: left ratio of the lesions was 2:1, and the mean degree of stenosis was 84.0%. The mean CSD was 5.9 mm, and the mean IAG was 60.4┬░ (Table 1).
First, we examined the effects of the PPLI on the CBR response. The PPLI group consisted of 39 patients. For the no PPLI group (n=12), the ╬öHR, ╬öSBP, and ╬öDBP were normally distributed (P value=0.200), and were therefore compared using StudentŌĆÖs t-tests. The mean ╬öHR, ╬öSBP, and ╬öDBP in the PPLI group (mean┬▒standard deviation) were 8.85┬▒1.61, 23.13┬▒2.51, and 13.41┬▒1.86, respectively, which were 74.60%, 64.34%, and 64.07% less than that of the no PPLI group, respectively (Table 2). Although the ╬öDBP, and not the ╬öHR and ╬öSBP, passed the LeveneŌĆÖs test for equality of variances, statistical analyses were performed with corrected degrees of freedom, and the differences of the means of all three variables were statistically significant with P values of 0.000 (Table 2).
Next, variables other than the CBR response that may have been influenced by the PPLI, that is, procedure time (mins) and length of ICU stay (days), were tested. These two variables were not normally distributed; therefore, the means of rank sums were compared using the Mann-Whitney U test to examine the effects of the PPLI. The procedure time was longer in the PPLI group than in the no PPLI group (28.19 [n=39] vs. 18.88 [n=12] minutes) (P=0.057), while the length of ICU stay was shorter in the PPLI group (20.01 [n=36] vs. 28.10 [n=5] days) (P=0.132). However, the Mann-Whitney U test compares the rank sums between the two groups; therefore, the actual size difference between the two groups cannot be stated. The differences in procedure time (P=0.057) and length of ICU stay (P=0.132) were not statistically significant (Table 3).
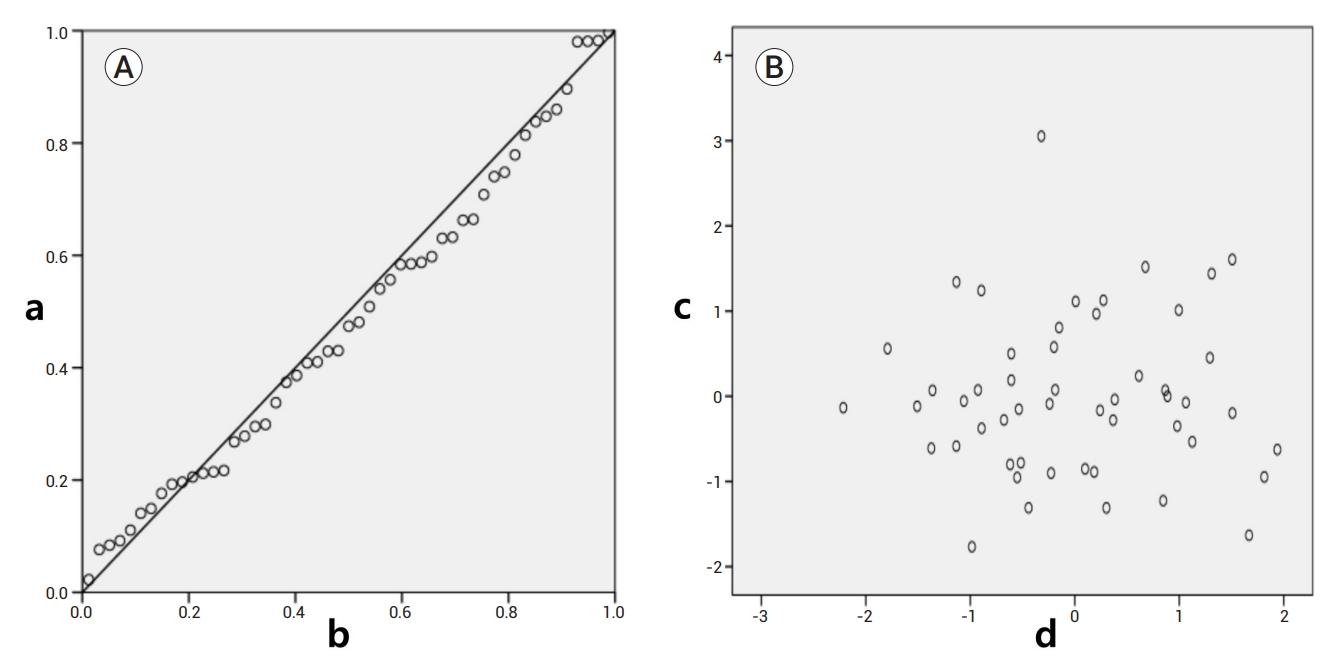
Linear regression analysis revealed that CSD (mm) was significantly associated only with the ΔDBP (Fig. 5) and not with the other parameters of the CBR response (P=0.027), and that these two variables were negatively correlated with a coefficient correlation of -0.893. The regression coefficient was approximately 17% (R2=0.166). The analysis of variance results indicated that the CBR response may be weaker with increasing distance from the CCA to the stenotic lesion, although the results were not significant (P=0.216). This was confirmed in the multiple regression analysis with a marginal statistical significance (P=0.056). The Durbin-Watson statistic was 1.279, indicating an absence of autocorrelation, and the variance inflation factor was 1.000, indicating the absence of multicollinearity (Table 4).
The correlations of patient and procedural variables with the carotid reflex response were analyzed with SpearmanŌĆÖs rank-order correlation and PearsonŌĆÖs correlation analyses. SpearmanŌĆÖs rank-order correlation analysis showed that the CSD (mm) was negatively correlated with all the CBR response parameters (╬öHR, ╬öSBP, ╬öDBP), and that these correlations were statistically significant (╬öHR [P=0.012], ╬öSBP [P=0.034], and ╬öDBP [P=0.036]). These parameters were negatively correlated in the PearsonŌĆÖs correlation analysis as well, although in terms of statistical significance, only the correlation with the ╬öDBP was marginally significant (P=0.056) (Table 5).
Although these results are insufficient to determine a complete linear relationship, subsequent studies with larger study populations could produce statistically significant results.
Atherosclerotic carotid artery stenosis causes 15-20% of all ischemic stroke cases, and CEA has been the treatment of choice for carotid artery stenosis for more than 60 years.
CAS was introduced approximately 30 years ago as an alternative to CEA due to its less-invasive nature and association with fewer surgical complications [8]. Since 2000, large clinical studies have compared CEA and CAS, including the Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) study, Endarterectomy versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S), Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE), International Carotid Stenting Study (ICSS), and Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) [12,15]. Although the findings of the these large-scale trials demonstrated that CEA was more favorable than CAS [9,22], these trials had several limitations, such as low proficiency of the physicians in the CAS group and a lack of routine use of EPDs. The CREST trial, the largest clinical trial in which highly proficient interventionists performed 95% of the surgeries and EPDs were used routinely, shed light on the utility of CAS, with comparable clinical outcomes to those of CEA, and recognized CAS as a useful tool for stroke prevention [5].
The carotid bulb is a dilation in the vasculature immediately distal to the CCA bifurcation, and is one of the major areas involved in atherosclerotic carotid stenosis [14,27]. The mechanosensitive nerve terminals of the carotid baroreceptors are located bilaterally in the adventitial layer of the carotid artery bifurcations [14,16]. The arterial baroreceptors sense changes in blood pressure or dilated blood vessels, leading to stretch-induced elevated uniaxial cytosolic Ca2+ levels and resulting in pressure-dependent action potential firing in the baroreceptor neurons. These neuronal signals are relayed to the cardiovascular control center in the brainstem for baroreflex regulation of blood pressure [14,18]. Such baroreceptor stimulation is related to hemodynamic depression, that is hypotension, bradycardia, or asystole, which occurs during CEA or CAS and is considered a baroreceptor reaction [6,14,16].
Prior to the introduction of CAS, the baroreceptor reflex during CEA was reported, and researchers have attempted to minimize the CBR response through direct lidocaine infiltration into the carotid bulb. However, many studies reported that lidocaine infiltration had minimal effects on CEA, presumably because baroreceptor sensitivity is weakened by the removal of atheromatous plaques; therefore, lidocaine infiltration had little impact on the already weakened baroreflex response [2,21,24]. Indeed, some researchers who conducted in vivo tests showed that the baroreceptor reflex is weakened by local anesthetic infiltration [3,24], which was explained as local anesthetic infiltration causing extramural neural blockage of the carotid sinus nerve [3,31].
In our study, we observed the CBR responseŌĆöreduced HR and BPŌĆöin response to stent deployment or balloon inflation during CAS in patients with carotid artery stenosis and investigated whether local anesthesia could attenuate the CBR response through a PPLI targeting the adventitia of the carotid bulb.
We examined statistically significant differences in the carotid reflex between the PPLI and no PPLI groups based on changes in HR and BP (ΔHR, ΔSBP, ΔDBP). We found that the changes in HR, SBP, and DBP were 74.60%, 64.34%, and 64.07% less, respectively, in the PPLI versus no PPLI group, suggesting that a PPLI significantly suppresses the CBR response (Table 2).
Furthermore, although not statistically significant, a PPLI was also associated with a shorter ICU stay and longer procedure times. However, we included a complicated case of a patient who had a seizure during PPLI administration, a case of severe carotid artery stenosis that required five rounds of ballooning, and a case that required additional procedures because the filter was obstructed by the stent and could not be removed. Hence, further data is needed to draw the conclusion that a PPLI lengthens the procedure time.
In addition, we examined factors other than if the PPLI could affect the CBR response, and observed that the degree of the CBR response declined with increasing CSD (mm). Further, these results are also in line with the finding of Suh et al. [30], that ŌĆ£the frequency of the carotid reflex differs according to the location of the atheromatous plaque.ŌĆØ Although statistically significant, these results were also marginally significant in the linear and multiple regression analyses. Therefore, the carotid reflex responded more sensitively when treating lesions closer to the CCA bifurcation [30]. Hence, we hypothesized that the infiltration of local anesthetic into the carotid bulb could enervate the carotid reflex response and could therefore more effectively minimize the carotid reflex during CAS procedures for proximal lesions from the CCA bifurcation.
Our study has some limitations. First, although we found statistically significant results showing that a PPLI reduced changes in vital signs caused by the CBR response, we did not investigate the specific aspects of the CAS procedure that could be enhanced by the suppression of the CBR response through the PPLI. Thus, studies with larger sample sizes are needed to focus on these aspects. Second, we examined factors other than the effect of a PPLI on the CBR response, although further research is needed to produce supplemental findings on more diverse factors that may influence the patients and procedures because carotid artery stenosis is an acquired lesion. In addition to being associated with various factors that could induce and exacerbate atherosclerosis, such as underlying diseases and lifestyle, it may be associated with the structural causes of carotid artery stenosis other than the CSD and IAG that were evaluated in this study. Third, as this was a retrospective study, the PPLI and no PPLI groups were not homogeneous. The sample size for each group differed, and the proportion of patients who underwent elective and emergency CAS also differed between the two groups. Hence, well-designed prospective studies, with larger study populations, homogeneous study groups, and double blinding, could potentially produce more statistically significant results.
The CBR may cause hemodynamic instability in patients being treated for carotid artery stenosis and thus complicate the procedure. Furthermore, the carotid baroreflex is a greater burden during CAS in which the anatomical structures are not damaged during the procedure. PPLI targeting the adventitia of the carotid bulb appears to help decrease the changes in HR and BP caused by carotid stent deployment and balloon inflation. Moreover, considering that carotid baroreflex sensitivity increases with decreasing distance to the stenotic lesion from the CCA bifurcation, a PPLI could help stabilize patients during procedures for stenotic lesions closer to the CCA.
Fig.┬Ā1.
Schematic diagram of the study design and number of included patients. M, male; F, female; n, number of patients; PPLI, percutaneous preprocedural lidocaine injection
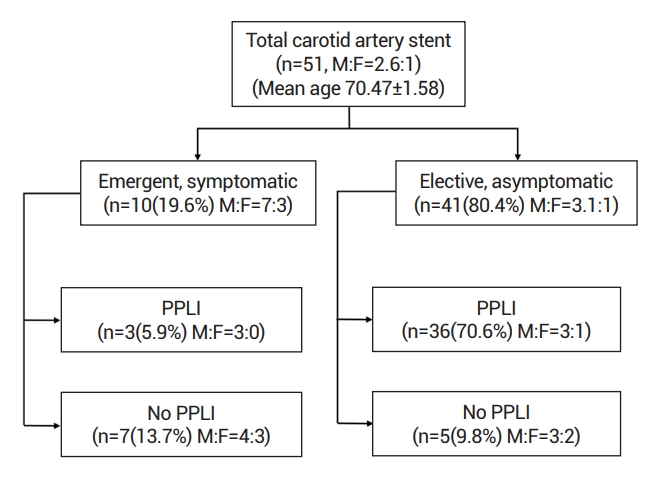
Fig.┬Ā2.
An illustration of PPLI procedure. Digital subtraction angiography in the lateral view. With Roadmap on, the needle is approached to the baroreceptor of the carotid bulb for lidocaine infiltration. A: Common carotid artery, B: external carotid artery, C: internal carotid artery, D: carotid bifurcation, marked on the skin with a radiopaque marker (mosquito forceps), E: the needle, and F: contrast showing the surgeonŌĆÖs hand. With Roadmap (including the radiopaque marker) on, the needle is approached using a real-time fluoroscopy guide, G: glossopharyngeal nerve, H: carotid sinus nerve connected to the carotid bulb, and I: baroreceptors in the carotid bulb. PPLI, percutaneous preprocedural lidocaine injection
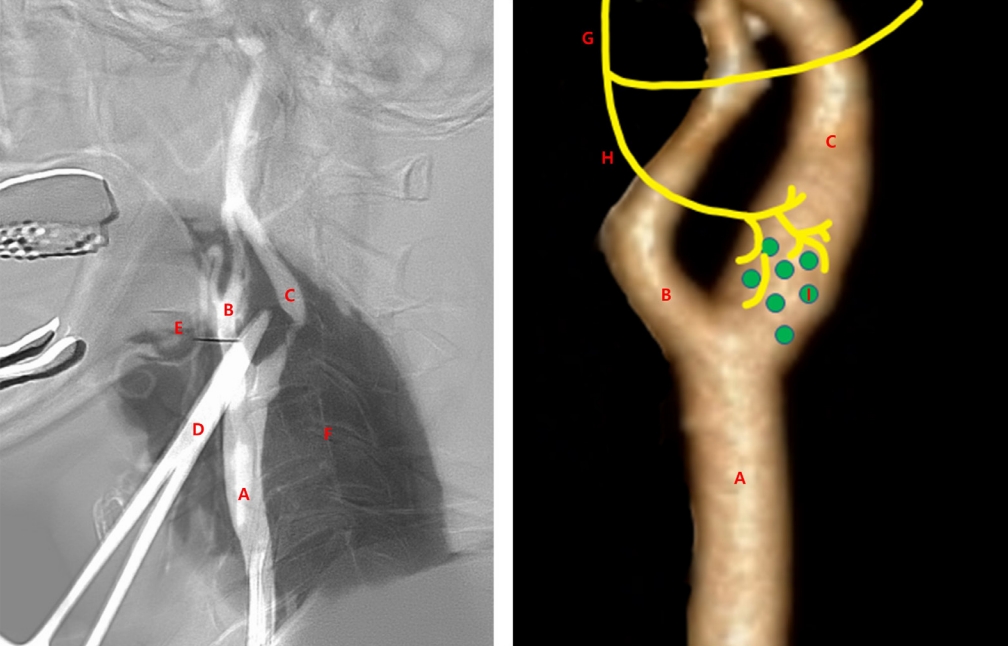
Fig.┬Ā3.
An illustration showing how to measure the distance from the carotid apex to the most stenotic lesion on the stenotic segment. A: common carotid artery, B: external carotid artery, C: internal carotid artery, and D: distance to be measured (mm).
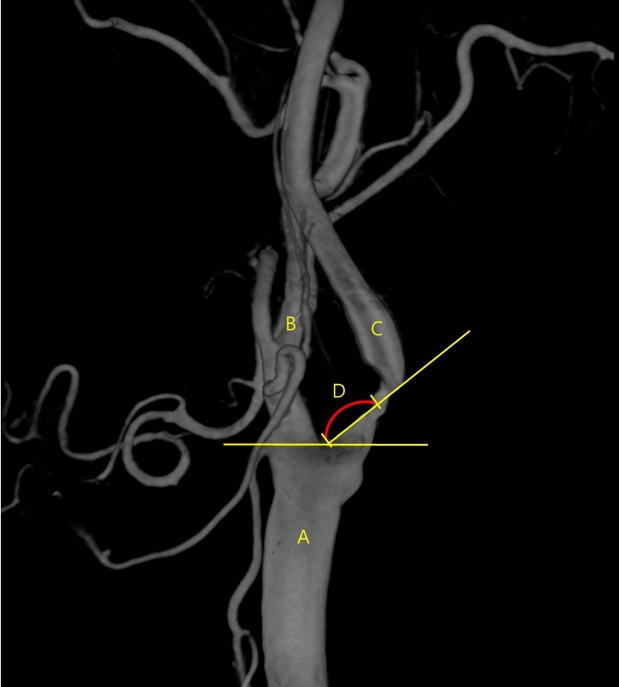
Fig.┬Ā4.
An illustration showing how to measure the angle at which the internal carotid artery branches off from the common carotid artery. A: common carotid artery, B: external carotid artery, C: internal carotid artery, and D: angle to be measured (degree).
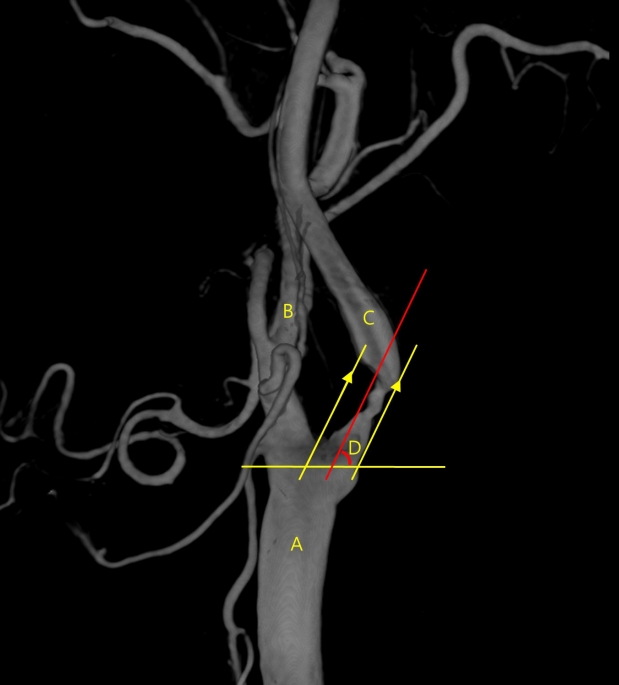
Fig.┬Ā5.
Normal P-P plot and scatter plot of the residuals of multiple regression. In the normal P-P plot (A), the distance from the CCA bifurcation to the most stenotic lesion (mm) is normally distributed, as it increases almost in line with the observed probability. In the scatter plot (B), the residuals are scattered randomly without a regular pattern, thus satisfying the independence and homoscedasticity. Although statistically insignificant, a marginal significance was observed (P value=0.056; dependent variable=diastolic blood pressure). a: expected cumulative probability, b: observed cumulative probability, c: regression standardized residuals, and d: standardized estimates. CCA, common carotid artery
Table┬Ā1.
Demographic characteristics of the patients
Table┬Ā2.
Association between percutaneous preprocedural lidocaine injection and carotid baroreflex (╬öHR, ╬öSBP, ╬öDBP) (StudentŌĆÖs t-test)
|
PPLI |
No PPLI |
LeveneŌĆĀ (P value) |
t-testŌĆĪ (P value) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Carotid reflex | N | Shapiro-Wilk test (P value) | Mean | Standard deviation | N | Shapiro-Wilk test (P value) | Mean | Standard deviation | ||
| ΔHR (bpm) | 39 | 0.011 (0.029)* | 8.85 | 1.61 | 12 | 0.158 (0.200)* | 34.67 | 30.25 | 0.000 | 0.000 |
| ΔSBP (mmHg) | 39 | 0.966 (0.200)* | 23.13 | 2.51 | 12 | 0.733 (0.200)* | 66.75 | 23.09 | 0.002 | 0.000 |
| ΔDBP (mmHg) | 39 | 0.960 (0.200)* | 13.41 | 1.86 | 12 | 0.960 (0.200)* | 37.33 | 15.19 | 0.234 | 0.000 |
Table┬Ā3.
Other variables that may be affected by PPLI besides the carotid baroreflex (Mann-Whitney test)
|
PPLI |
No PPLI |
P value (two-tail) | |||||
|---|---|---|---|---|---|---|---|
| N | Mean rank | Sum of ranks | N | Mean rank | Sum of ranks | ||
| Procedure time (min) | 39 | 28.19 | 1099.50 | 12 | 18.88 | 226.50 | 0.057 |
| ICU time (days) | 36 | 20.01 | 720.50 | 5 | 28.10 | 140.50 | 0.132 |
Table┬Ā4.
Association between carotid baroreflex (ΔHR, ΔSBP, ΔDBP) and variables besides PPLI (linear and multiple regression analyses)
HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; PPLI, preprocedural percutaneous lidocaine injection; VIF, variance inflation factor; ICA, internal carotid artery; ANOVA, analysis of variance
Balloon number: number of balloon inflations
Stent number: number of deployed stents
Table┬Ā5.
Monotonic and linear relationship between carotid baroreflex (ΔHR, ΔSBP, ΔDBP) and variables besides PPLI
REFERENCES
1. Abbott AL, Paraskevas KI, Kakkos SK, Golledge J, Eckstein HH, Diaz-Sandoval LJ, et al. Systematic review of guidelines for the management of asymptomatic and symptomatic carotid stenosis. Stroke. 2015 Nov;46(11):3288-301.


2. Ajduk M, Tudori─ć I, ┼Āarlija M, Pavi─ć P, Oremu┼Ī Z, Held R, et al. Effect of carotid sinus nerve blockade on hemodynamic stability during carotid endarterectomy under local anesthesia. J Vasc Surg. 2011 Aug;54(2):386-93.


3. Al-Rawi PG, Sigaudo-Roussel D, Gaunt ME. Effect of lignocaine injection in carotid sinus on baroreceptor sensitivity during carotid endarterectomy. J Vasc Surg. 2004 Jun;39(6):1288-94.


4. Bonati LH, Kakkos S, Berkefeld J, de Borst GJ, Bulbulia R, Halliday A, et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur Stroke J. 2021 Jun;6(2):I-XLVII.


5. Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016 Mar;374(11):1021-31.



6. Cao Q, Zhang J, Xu G. Hemodynamic changes and baroreflex sensitivity associated with carotid endarterectomy and carotid artery stenting. Interv Neurol. 2015 Jan;3(1):13-21.



7. Cayne NS, Faries PL, Trocciola SM, Saltzberg SS, Dayal RD, Clair D, et al. Carotid angioplasty and stent-induced bradycardia and hypotension: Impact of prophylactic atropine administration and prior carotid endarterectomy. J Vasc Surg. 2005 Jun;41(6):956-61.


8. De Rango P, Parlani G, Verzini F, Giordano G, Panuccio G, Barbante M, et al. Long-term prevention of stroke: A modern comparison of current carotid stenting and carotid endarterectomy. J Am Coll Cardiol. 2011 Feb;57(6):664-71.

9. Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. TLancet Neurol. 2008 Oct;7(10):893-902.

10. European Stroke Organisation, Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Cl├®ment D, et al. ESC guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011 Nov;32(22):2851-906.


11. Fox AJ, Eliasziw M, Rothwell PM, Schmidt MH, Warlow CP, Barnett HJ. Identification, prognosis, and management of patients with carotid artery near occlusion. AJNR Am J Neuroradiol. 2005 Sep;26(8):2086-94.


12. George JC, White CJ. Carotid artery stenting: Lessons from CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial). JACC Cardiovasc Interv. 2010 Sep;3(9):988-90.

13. Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The CAPTURE registry: Results of carotid stenting with embolic protection in the post approval setting. Catheter Cardiovasc Interv. 2007 Feb;69(3):341-8.


14. Gupta R, Horowitz M, Jovin TG. Hemodynamic instability after carotid artery angioplasty and stent placement: A review of the literature. Neurosurg Focus. 2005 Jan;18(1):e6.

15. Komotar RJ, Starke RM, Connolly ES. Carotid endarterectomy vs endovascular stenting: Recent results from ICSS and CREST. Neurosurgery. 2010 Jun;66(6):N12-3.
16. Lau EO, Lo CY, Yao Y, Mak AF, Jiang L, Huang Y, et al. Aortic baroreceptors display higher mechanosensitivity than carotid baroreceptors. Front Physiol. 2016 Aug;7:384.



17. Leisch F, Kerschner K, Hofmann R, Steinwender C, Grund M, Bibl D, et al. Carotid sinus reactions during carotid artery stenting: Predictors, incidence, and influence on clinical outcome. Catheter Cardiovasc Interv. 2003 Apr;58(4):516-23.


18. Levy M, Pappano A, Berne R. Cardiovascular physiology. Philadelphia, PA: Mosby Elsevier; 2007.
19. Lim HH, Ho KM, Choi WY, Teoh GS, Chiu KY. The use of intravenous atropine after a saline infusion in the prevention of spinal anesthesia-induced hypotension in elderly patients. Anesth Analg. 2000 Nov;91(5):1203-6.


20. Lin PH, Zhou W, Kougias P, El Sayed HF, Barshes NR, Huynh TT. Factors associated with hypotension and bradycardia after carotid angioplasty and stenting. J Vasc Surg. 2007 Nov;46(5):846-53; discussion 853-4.


21. Maher CO, Wetjen NM, Friedman JA, Meyer FB. Intraoperative lidocaine injection into the carotid sinus during endarterectomy. J Neurosurg. 2002 Jul;97(1):80-3.


22. Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: Results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008 Oct;7(10):885-92.


23. McKevitt F, Randall M, Cleveland T, Gaines P, Tan K, Venables G. The benefits of combined anti-platelet treatment in carotid artery stenting. Eur J Vasc Endovasc Surg. 2005 May;29(5):522-7.


24. Mourikis D, Chatoupis K, Katsenis K, Vlahos L, Chatziioannou A. Percutaneous injection of lidocaine within the carotid body area in carotid artery stenting: An ŌĆ£old-newŌĆØ technique. Cardiovasc Intervent Radiol. 2008 Jul-Aug;31(4):709-12.



25. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991 Aug;325(7):445-53.


27. Pujia A, Rubba P, Spencer MP. Prevalence of extracranial carotid artery disease detectable by echo-Doppler in an elderly population. Stroke. 1992 Jun;23(6):818-22.


28. Qureshi AI, Luft AR, Sharma M, Janardhan V, Lopes DK, Khan J, et al. Frequency and determinants of postprocedural hemodynamic instability after carotid angioplasty and stenting. Stroke. 1999 Oct;30(10):2086-93.


29. Stallard S, Prescott S. Postoperative urinary retention in general surgical patients. Br J Surg. 1988 Nov;75(11):1141-3.



30. Suh D, Kim J, Kim E, Kim J, Shin JH, Hyun D, et al. Carotid baroreceptor reaction after stenting in 2 locations of carotid bulb lesions of different embryologic origin. AJNR Am J Neuroradiol. 2012 May;33(5):977-81.



-
METRICS

-
- 0 Crossref
- 0 Scopus
- 771 View
- 32 Download
- ORCID iDs
-
Tae Joon Park

https://orcid.org/0000-0001-7509-7060 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print