Transcranial Doppler emboli monitoring for stroke prevention after flow diverting stents
Article information
Abstract
Objective
Flow diverting stents (FDS) are increasingly used for the treatment of intracranial aneurysms. While FDS can provide flow diversion of parent vessels, their high metal surface coverage can cause thromboembolism. Transcranial Doppler (TCD) emboli monitoring can be used to identify subclinical embolic phenomena after neurovascular procedures. Limited data exists regarding the use of TCDs for emboli monitoring in the periprocedural period after FDS placement. We evaluated the rate of positive TCDs microembolic signals and stroke after FDS deployment at our institution.
Methods
We retrospectively evaluated 105 patients who underwent FDS treatment between 2012 and 2016 using the Pipeline stent (Medtronic, Minneapolis, MN, USA). Patients were pretreated with aspirin and clopidogrel. All patients were therapeutic on clopidogrel pre-operatively. TCD emboli monitoring was performed immediately after the procedure. Microembolic signals (mES) were classified as “positive” (<15 mES/hour) and “strongly positive” (>15 mES/hour). Clinical stroke rates were determined at 2-week and 6-month post-operatively.
Results
A total of 132 intracranial aneurysms were treated in 105 patients. TCD emboli monitoring was “positive” in 11.4% (n=12) post-operatively and “strongly positive” in 4.8% (n=5). These positive cases were treated with heparin drips or modification of the antiplatelet regimen, and TCDs were repeated. Following medical management modifications, normalization of mES was achieved in 92% of cases. The overall stroke rates at 2-week and 6-months were 3.8% and 4.8%, respectively.
Conclusions
TCD emboli monitoring may help early in the identification of thromboembolic events after flow diversion stenting. This allows for modification of medical therapy and, potentially, preventionf of escalation into post-operative strokes.
INTRODUCTION
Flow diversion is becoming an increasingly used strategy for the treatment of complex intracranial aneurysms [4,6,12,15,17]. The pipeline embolization device (PED, Medtronic, Minneapolis, MN, USA) is a flow diverting stent that provides up to 35% metal coverage over the parent vessel. It works by disrupting intra-aneurysmal blood flow, therefore promoting stasis and aneurysmal thrombosis. Over time, the stent becomes endothelialized resulting in aneurysm neck obliteration and a durable aneurysm cure [6,7].
The high metal surface of a PED is associated with thrombo-embolic complications [3]. Dual antiplatelet agents (aspirin in combination with one of the P2Y12 inhibitors - clopidogrel, prasugrel, or ticagrelor) have been used to inhibit platelet activation [10]. Aspirin and clopidogrel response assays can be used before and after interventions to confirm platelet inhibition. However, the association between extent of platelet inhibition and thromboembolic events is not clear. As such, there is significant variability in the practice patterns of neurointerventionalists in the use of platelet inhibition assays prior to deployment of PEDs [10,11].
In this study, we analyzed the use of transcranial Doppler (TCD) monitoring to evaluate microembolic events after flow diversion [1]. Intracranial microembolic signals (mES) are predictive of strokes both de novo [9,22] and following neurointerventional procedures [13,19]. For instance, mES are higher in patients with carotid disease and cardioembolic sources when compared to healthy individuals.8) Additionally, microemboli monitoring has been used to minimize stroke during and after carotid endarterectomy and cardiac valve surgeries [16]. No data exists regarding the use of TCDs for emboli monitoring in the periprocedural period after flow diverting stent placement. In this study, we present our institutional experience with microemboli monitoring and stroke rates after pipeline embolization of intracranial aneurysms.
MATERIALS AND METHODS
Study design and population
Medical records of our institute were retrospectively reviewed from 110 consecutive patients with intracranial aneurysms treated with the PED between 2012 and 2016. Demographic data and clinical data were analyzed.
Inclusion criteria included all patients with unruptured, or previously coiled aneurysms treated with Pipeline stent (Medtronic, Minneapolis, MN, USA). To minimize confounding variables in the study, we excluded patients who underwent PED treatment for aneurysmal subarachnoid hemorrhage, pseudoaneurysms, or residual aneurysms that were previously stented. Based on this criterion, we excluded five patients and analyzed a total of 105 patients.
Statistical analysis was performed with Prism 9.0.1 software (GraphPad Software, Inc., San Diego, CA, USA). For continuous variables the Mann-Whitney test was used. For categorical variables, chi-square test was employed. A p value <0.05 was considered significative.
Treatment protocol
Our PED deployment protocol has been described previously [15]. Patients were pre-treated with aspirin (81 mg) and clopidogrel (75 mg) for at least 10 days prior to treatment. Therapeutic levels were determined using the Accumetrics VerifyNow P2Y12 assay. P2Y12 reaction units (PRU) <208 were considered therapeutic on clopidogrel. Patients with PRU >208 were considered clopidogrel-non-responders and started on ticagrelor. Baseline and post-clopidogrel/ticagrelor treatment PRU values were used to calculate % platelet inhibition: 1-(treatment PRU/baseline PRU). The platelet inhibition goal was 30-80% activity.
All patients were adequately heparinized during the procedure with activated clotting times greater than 280 seconds.
Patients were kept on a regimen of aspirin for life, and clopidogrel or ticagrelor for at least 6 months. PEDs were deployed using a standard triaxial system. Control digital subtraction angiography was performed immediately following PED placement. A follow-up angiography was performed at 6 months post-treatment.
Transcranial Doppler studies
TCD emboli monitoring was performed 6 hours and prior to 24-hours post-operatively. The Transcranial Power M-mode transcranial Doppler system (PMD 100 or ST3; Spencer Technologies, Seattle, WA, USA) with a 2-MHz digital Doppler was used [21]. All exams were performed by registered vascular sonographers specializing in neurovascular sonography. Criteria for mES identification were: 1) characteristic acoustic properties, 2) short duration (<0.3 ms), and 3) random appearance during cardiac cycle. Emboli monitoring was performed continuously for at least 20 minutes in the arterial segment distal to the aneurysm.
Management of patients with positive TCD emboli
All cases with positive mES underwent repeated monitoring withing 6-24 hours. If the initial two scans showed <15 mES/hr, no further studies were performed. If “strongly positive” rates (>15 mES/hr) were noted, TCDs were repeated within 6-hours after medical treatment modification consisting of either heparinization, additional clopidogrel load, or pharmacological switch from clopidogrel to ticagrelor. TCDs were repeated until two stable scans showed <15 mES/hr.
RESULTS
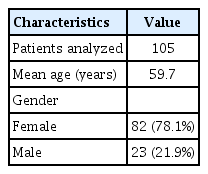
Between October 2012 and July 2020, 110 patients were treated with PEDs for unruptured or previously coiled intracranial aneurysms. Aneurysmal subarachnoid hemorrhage (SAH) and pseudoaneurysms were excluded (n=5). Based on these criteria, we studied 105 patients with 132 aneurysms. The mean patient age was 59.7 years, with the majority (78.1%) being female (Table 1). The mean aneurysm size was 7.8±6.4 mm. The paraclinoid region was the most frequent aneurysm location (Table 2). Two aneurysms in the petrous segment were treated due to intradural aneurysmal extension.
Most cases involved deployment of a single PED (N=93, 88.6%). In twelve cases (11.4%), two PEDs were deployed. Adjunctive aneurysm coiling was performed in fifteen cases (14.3%) (Table 3), and was associated with a higher incidence of positive TCD results (not statistically significant).
Table 4 summarizes the TCD emboli monitoring results from all cases along with associated aneurysm size, number of PEDs deployed, treatment PRU as well as % platelet inhibition. Inadequate transcranial windows prevented TCD emboli monitoring in seven cases (6.5%). Patients with positive TCD emboli (N=12, 11.4%) had an average pre-procedure PRU of 144.1±46.6 and % platelet inhibition of 38.2±20.1%. Patients with negative TCD emboli (N=86, 81.9%) had an average pre-procedure PRU of 113.2±59.0 with a % platelet inhibition of 58.3±20.6%. Significantly less % platelet inhibition was evodemt in patients with positive mES when compared to patients with negative mES (38.2±20.1% vs 58.3±20.6%, P<0.02). Positive TCD results did not correlate with aneurysm size or number of PEDs deployed (Tables 3 and 4). Table 4 also shows that, after medical interventions were initiated to prevent on-going mES in patients with positive TCDs, no strokes were evident upon follow-up.
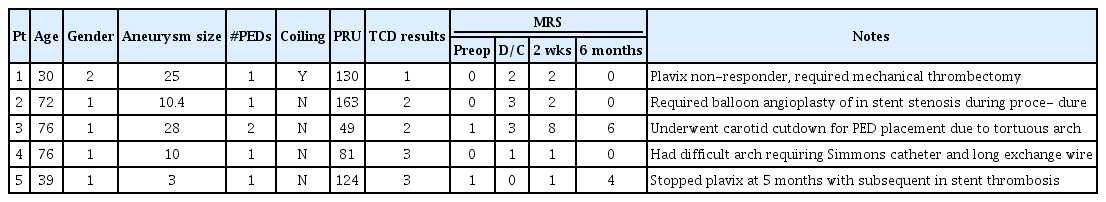
Table 5 presents the 12 cases of positive mES after PED deployment along with the patient demographics, aneurysm size and treatment details. The magnitude of embolic signals ranged between 3 to 303 mES/hr. Patients with embolic signals less than 15 mES/hr were closely followed with repeat TCDs only and no additional monitoring was done in cases when repeat TCDs showed no further emboli.
In cases of strongly positive TCD signals (>15 mES/hr), patients were either started on heparin and/or reloaded with clopidogrel or switched to ticagrelor. TCDs were again checked within 6-hours to confirm cessation of embolic signals. In one case (patient 10), embolic signals persisted despite changes in antiplatelet regimen, and an in-stent thrombosis evolved necessitating mechanical thrombectomy. Of note, this patient was a responder to clopidogrel prior to the stent placement. Surprisingly, he transitioned to be a non-responder on immediate post-operative testing. The patient was ultimately placed on ticagrelor and coumadin which aborted further mES. This patient’s outcome was favorable with a full recovery and no neurologic deficits.
Table 6 presents the five strokes (4.8%) that occurred in the 105 patients in this study. Patient 1 as described above was a clopidogrel non-responder and had persistent TCD emboli until stabilized on ticagrelor and coumadin. The next two patients had neurologic deficits in the immediate post-operative period despite negative TCDs. Patient 2 had a posterior putaminal stroke relating to a thrombotic event linked to balloon angioplasty of in-stent stenosis, but made a full recovery at 6-months follow-up. Patient 3 died within a 2-week period from a severe gastrointestinal bleeding. Of note, this patient had a very tortuous arch anatomy and underwent flow diverting stents (FDS) of a giant cavernous segment aneurysm via a cervical carotid cut down. Patient 4 had inadequate temporal windows for TCD monitoring and recovered from endovascular treatment with left hemiparesis relating to an embolic event from the use of a Simmons catheter to navigate a difficult Type III aortic arch. Finally, patient 5 had a thrombotic complication at 5-months after she stopped clopidogrel and aspirin with resultant stent thrombosis. This patient had no TCD windows.
DISCUSSION
PEDs are widely used for complex and wide-necked intracranial aneurysms [4-6,12,15,17]. They promote flow stagnation, aneurysmal thrombosis, and parent vessel remodeling through endothelialization of the stent. Both thrombotic and embolic complications can occur after PED deployment [3,11]. Flow stagnation at branch vessels covered by PEDs can cause thrombosis and occlusion of the perforating arteries [18]. Embolic complications can occur with dislodgement of platelet aggregates from the stent surface causing distal vessel occlusion.
TCD emboli monitoring has been shown to prevent ischemic complications during cardiac surgery [16] and carotid endarterectomy procedures [20,21,23]. Similar results have also been shown for emboli monitoring after intracranial aneurysm coiling [13,19]. These studies demonstrate that reduced microembolic events is associated with reduced ischemic events. Schubert et al. showed that early heparinization was associated with reduced TCD emboli as well as reduced neurologic deficits post-procedure [19].
In this study, we found that 11.4% of cases (12 of 105 patients) shower positive mES after PED deployment. In 5 of 12 patients, the emboli resolved with repeat testing and no intervention. In 6 of 12 patient’s additional medical interventions (including further heparinization, additional clopidogrel administration, or change in antiplatelet agent) were needed to reduce the microembolic events. In one of the twelve patients, who was clopidogrel-resistant and ticagrelor super-responder, mechanical thrombectomy was needed due to rapid in-stent thrombosis. In this patient, TCD emboli monitoring was pivotal in establishing the appropriate antiplatelet regimen. All twelve patients with microemboli after PED deployment had cessation of TCD emboli prior to hospital discharge and were neurologically intact at 30-days. Comparison of this group of patients to the group with no TCD emboli demonstrated that % platelet inhibition was the only significantly different variable predicting ischemic events.
Our study suggests that close monitoring of % platelet inhibition peri-procedurally results in reduced ischemic events [14]. The routine use of platelet function assays is debated in the neurovascular community [10]. Approximately 11.5% of neuro-interventionalists do not routinely perform platelet function testing after PED deployment. This likely reflects the absence of an established association between stroke and degree of platelet inhibition. For instance, Heller et al. reported no association between strength of antiplatelet therapy and procedural embolism after PED deployment [11]. Whereas Brasiliense et al. and Delgado-Almandoz et al. showed that the preprocedure PRU values were most predictive of thromboembolic events.
Finally, reduction in mES is associated with reduced risk of ischemic events and stroke [9]. The overall stroke rate in our study was 3.8% at two-weeks and 4.8% at six-months, somehow comparable to the 5.6% stroke and mortality rate in the PUFs trial [2]. We believe that our stroke rates could have been higher if no TCD emboli monitoring had been available, since abortion of ongoing embolic events was achieved in most cases after positive mES were detected.
CONCLUSIONS
TCD emboli monitoring represents a powerful non-invasive tool for early identification of thromboembolic events after flow diversion stenting. Early detection of microembolic signals allows for modification of post-operative medical therapy and, potentially, prevention of post-operative stroke.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.