 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(4); 2023 > Article |
|
Abstract
Objective
Chronic subdural hematoma (CSDH) is a neurological complication following clipping surgery. However, the natural course and ideal approach for the treatment of clipping-related-CSDH (CR-CSDH) have not been clearly established. We aimed to investigate the course of CR-CSDH using chronological radiological findings.
Methods
We performed a retrospective analysis of 28 (3.8%) patients who developed CSDH among 736 patients who underwent surgical clipping using pterional approach for unruptured aneurysms at our institution between December 2010 and December 2018. Patients underwent follow-up CT scan 6-8 weeks after clipping surgery and decision to pursue surgical intervention rests upon the patientŌĆÖs symptom based on the MarkwalderŌĆÖs grading scale (MGS) and numeric rating scale (NRS).
Results
Of the 28 patients, 3 patients (10.7%) underwent surgery, while 25 (89.2%) showed spontaneous resolution of CR-CSDH. Eighteen patients (64.2%) had mild headache with MGS of 0-1. The mean maximum hematoma volume was 41.9┬▒30.9 ml (5.8-135 ml), and 26 patients (92.8%) had homogeneous hematoma. The mean time to hematoma resolution was 126.7┬▒52.9 days (46-228 days). Comparing group of CR-CSDH volume Ōēź43 ml or a midline shift Ōēź5 mm, the difference in presence of linear low-density area (p=0.002) and age (p=0.026) between the conservative and operative groups were found to be statistically significant.
Clipping surgery is still an essential method for the treatment of unruptured aneurysms, along with endovascular procedures. Even though surgical techniques for clipping surgery have been remarkably improved with regards invasiveness, there are still inherent surgical complications such as chronic subdural hemorrhage (CSDH), surgical wound infection, and cerebrospinal fluid (CSF) leakage [5]. Among these complications, CSDH following clipping surgery is not a rare complication, occurring in 2-5% of cases, causing a burden of additional surgical treatment [6,17].
Although many studies have been conducted on risk factors related to clipping-related-CSDH (CR-CSDH) [12,16], the natural course and ideal approach for the treatment of CR-CSDH have not been clearly established. However, decision for patients and surgeons to consider subsequent surgery for CR-CSDH is burdensome. We, therefore, aimed to investigate the natural course of CR-CSDH through chronological radiologic findings and to determine the optimal management strategy.
After obtaining institutional review board approval, we conducted a retrospective analysis of a prospectively maintained database for 736 patients diagnosed with unruptured cerebral aneurysm who underwent clipping surgery between December 2010 and December 2018 at our institution. Since January 2011, we have prospectively followed up patients who developed CR-CSDH using our study protocol. The proposed criteria for CR-CSDH are postoperative isodense or hyperdense crescent-shaped extra-axial collections in the frontoparietal area rather than hypodense subdural hygroma based on computed tomography (CT) scans.
Twenty-eight patients were included in the present study according to the following criteria: 1) surgically clipped unruptured cerebral aneurysm using a pterional approach; 2) spontaneously developed CR-CSDH with absence of trauma history during follow-up period after discharge; 3) no radiologic evidence of CSDH or subdural hygroma before surgery; and 4) no medication (statin, tranexamic acid, steroid, and angiotensin-converting enzyme [ACE] inhibitors) that may have an effect on the resolution of CSDH. Of the 736 patients, 21 were excluded for non-pterional approach, 5 for preoperative confirmed CSDH and 2 for steroid use, leaving 708 patients. Of the 708 patients, 28 patients with postoperative CSDH were included in the study, none of whom had a history of trauma during the observation period. The following baseline characteristics were analyzed at admission: sex; age; hypertension (HTN); diabetes mellitus (DM); smoking; alcohol consumption; cerebrovascular disease; and use of any antithrombotic medications including aspirin, clopidogrel, warfarin, cilostazol, triflusal, dipyridamole and direct oral anticoagulant.
To reduce inter-surgeon variability, we only included patients who received treatment and had regular follow-up with one neurosurgeon (C.Y.L). There were no differences in the surgical procedures or postoperative management among patients.
In general, patients without wound infection or other postoperative complications after clipping surgery were discharged on the third to seventh postoperative day (POD), and the first outpatient follow-up was performed 2 weeks after discharge. The first follow-up CT scan was performed approximately 6-8 weeks after clipping surgery if the patient had no symptoms.
After the radiologic diagnosis of CR-CSDH in the first follow-up CT scan, patients underwent a structured interview at every outpatient clinic visit. At the end of the interview, patients were assigned a MarkwalderŌĆÖs grading scale (MGS), which describes the clinical pattern from those used in a previously reported series of CSDH (Table 1) [13]. The patientŌĆÖs neurological state at presentation was trichotomized according to the MGS score (asymptomatic=0, mild-symptomatic=1, neurosurgical-symptomatic Ōēź2). And the patientsŌĆÖ pain was also evaluated using a numeric rating scale (NRS), a previously applied 11-point numeric scale for headache assessment [3]. A headache after clipping surgery is often expressed as mild and aggravated when the mouth is open (NRS <4); limited to headaches that do not interfere with daily life and are treatable with acetaminophen. Therefore, we defined NRS Ōēź4 as a significant headache affected by CR-CSDH. Finally, the patients with MGS >1 or NRS Ōēź4 should be considered for surgical intervention. Patients with an MGS Ōēż1 and an NRS <4 points were followed under close observation with serial CT scans every 30 days from diagnosis without surgical intervention. In addition, in some patients with CR-CSDH volume Ōēź43 ml or a midline shift Ōēź5 mm, outpatient visits with CT scans were recommended at least every 2 weeks (Fig. 1).
Radiologic evaluation was performed using a CT scan with an identical imaging protocol, which consisted of a 512├Ś512 matrix with 4 mm axial slices obtained from a SIEMENS 256-slice SOMATOM Force CT scanner (Henkestr, Germany). The following radiologic characteristics were analyzed with every outpatient CT scan: hematoma volume, hematoma location, midline shifting, mean Hounsfield units (HU) of hematoma, characteristics of CR-CSDH, and presence of a linear low-density area between the hematoma and brain cortex.
To measure the volume of the subdural hematoma, volumetric studies were performed using the axial planes of the CT scan with a section thickness of 5 mm. PiViewSTAR (Infinitt Co., Ltd., Seoul, Korea), a picture archiving and communication system (PACS), was used to measure the area of the subdural hematoma in each axial slice by manually outlining the region of interest. The maximum midline shift was measured as the maximum length of deviation from the vertical midline of the skull to the septum pellucidum. Mean Hounsfield units were measured using the average figure in the single axial section of CT images, which showed the maximal thickness of CR-CSDH between the outer margin of the cortex and the inner table of the skull. The characteristics of CR-CSDH on CT scans were classified according to Nomura et al.: (A) high-density, (B) iso-density, (C) low-density, (D) mixed-density, and (E) layering [15]. And according to Nomura et al., as mixed-density and layering CSDH indicate recent bleeding, characteristics of CR-CSDH is divided into two group: homogenous-type (A, B, C) and heterogenous-type (D, E). The presence of a linear low-density area between the inner membrane of the hematoma and the outer margin of the cortex which suggesting remained CSF beneath the hematoma (Fig. 2), as reported by Hiroshi et al., was analyzed in the axial plane of the CT scan [4].
The radiologic confirmation of CR-CSDH and volumetry studies using CT scans were performed by two neurosurgeons (J.H.K, S.C.K) blinded to the clinical course of the patients. Moreover, preoperative radiographic data including the degree of brain atrophy were reviewed. The degree of atrophy was measured using a previously described grading method [10].
Patient symptoms were identified through telephone surveys at intervals of one week for patients without volume reduction of the subdural hematoma. When the NRS score deteriorated by >3 points, or MGS by >1 point, additional neurologic and radiologic examinations were conducted immediately through outpatient visits or emergency room visits (Fig. 1). The decision to pursue surgical intervention rests upon the presence of the patientŌĆÖs neurological state based on the MGS and NRS at the outpatient clinic or emergency room, not on hematoma volume or midline shift. Surgical intervention was performed in patients with MGS >1 or NRS Ōēź4. The endpoints were radiologic evidence of complete spontaneous resolution of CR-CSDH on CT scan, clinical evidence of MGS and an NRS score of 0 points, or surgical intervention of CR-CSDH.
To investigate the variables of patients requiring surgery among the overall CR-CSDH patients, the patients were divided into two groups: nonoperative CR-CSDH (n=25) and operative CR-CSDH (n=3). A comparison between the two groups was performed for sex, age, DM, HTN, brain atrophy, and the presence of low-density areas.
Additionally, to evaluate the variables predicting the risk of surgical or conservative treatment of CR-CSDH with relative large volume and midline shift [7], we identified 12 patients with CR-CSDH volume Ōēź43 ml or midline shift Ōēź5 mm. Nine of the 12 patients underwent conservative treatment (conservative group) and the remaining three patients underwent surgery (surgical group). The conservative and surgical groups were compared by sex, age, DM, HTN, brain atrophy, MGS score, NRS score, and presence of linear low-density areas. In addition, to investigate the chronological radiographic findings of nonoperative CR-CSDH, the volume, HUs, and midline shifting of the CT scan performed at each outpatient visit were analyzed. Our statistical analysis utilized SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Continuous data are expressed as mean┬▒standard deviation (SD) and were compared using an independent t-test or Mann-Whitney U test. Categorical data are expressed as frequencies and percentages, and the proportion difference between the two groups was compared using the chi-square test. A decrease in value of the follow-up data in the nonoperative group was analyzed using repeated measures ANOVA based on the mixed model. Results with p values <0.05 were considered statistically significant.
Among 736 patients who underwent clipping surgery, 28 (3.8%) were included in the study. In a total 28 patients with CR-CSDH, twenty (71.4%) were male. The mean age of the patients was 62.5┬▒9.11 years (range 45-75).
All hematomas occurred on the ipsilateral craniotomy side. The most common hematoma location was in the frontoparietal region in 19 patients (67.8%). Twenty-six patients (92.8%) showed homogenous-type hematoma during the follow-up period. Only two patients (7.2%) had heterogeneous type hematomas, all of which were the laminar type (Table 2). The mean maximum hematoma volume during the follow-up period was 41.9 ml (range 5.8-135 ml). In addition, a midline shift was seen in 13 patients (46.4%) and mean maximum midline shifting was 5.46 mm (range 2.78-9.69 mm).
Spontaneous resolution of hematoma was observed in 25 patients (89.2%), who were asymptomatic (n=7) or mildly symptomatic (n=18) with a NRS score of <4 points and an average score of 1.53 (range, 1-3). The average interval between aneurysm clipping and hematoma resolution was 126.7 days (range 46-228 days) and average interval to maximum hematoma volume is 60.2 days (range 39-139 days). Table 3 presents the detailed radiological and clinical findings. As the amount of hematoma spontaneously decreased, the Hounsfield units also decreased (Table 4). Meanwhile, a volume increase of the hematoma during the follow-up period was observed in six patients (21.4%), and two of the six patients presented with radiologic deterioration again at the third follow-up. Also, a midline shift increase was observed in four patients (14.3%) in the second follow-up CT scan, and one of four patient presented radiologic aggravation at third follow-up. However, none of the patients showed symptom aggravation despite radiological aggravation. Therefore, we continued observation without surgical intervention. All six patients showed resolution of the hematoma.
Neurosurgical-symptomatic (MGS Ōēź2) CR-CSDH occurred in three patients, with hematoma volumes of 103 ml, 139 ml, and 68.7 ml, and midline shifts of 7.22 mm, 7.54 mm, and 7.7 mm, respectively. The interval between aneurysm clipping and burr-hole trephination was 44 days (range: 35-47 days). None of the patients showed impairments in daily living, permanent neurologic deficits, or CSDH recurrence.
Among overall CR-CSDH, the presence of a linear low-density area was significantly higher in the nonoperative group than in the operative group (p=0.002). When comparing CR-CSDH volume Ōēź43 ml or midline shift Ōēź5 mm, the presence of linear low-density areas (p=0.002) and age (p=0.026) showed statistically significant difference between the conservative and surgical groups. Additionally, brain atrophy, sex, HTN, DM, volume, midline shifting, and Hounsfield units did not show statistically significant differences (Table 5).
A 57-year-old man underwent surgical clipping for an unruptured right middle cerebral artery (MCA) bifurcation aneurysm. The patient was taking medication for hypertension and pontine infarction. Antiplatelet therapy was stopped for more than 5 days before surgery. The surgery was performed using the ipsilateral pterional approach, and there were no events during the surgery. The patient was discharged without any specific symptoms after surgery. A CT scan taken on the 44th POD showed high-density CSDH in the frontoparietal area (Fig. 3A). The hematoma volume was 78.1 ml, maximum thickness was 17.27 mm, HUs was 58.14, and midline shift was 8.34 mm. The patient was suffering from mild symptoms (MGS=1) and headache symptoms (NRS=2). We recommended that the patient attend an outpatient clinic for 2 weeks and instructed to visit the emergency department whenever neurological symptoms occurred.
CT scans on 58th and 72th POD showed no significant difference except for a slight increase in volume and then decrease again, and there were no neurological symptoms other than mild headache, so we decided to continue outpatient follow-up.
On the 86th day postoperative CT, the hematoma became hypo-dense and significantly decreased in volume, and the symptoms completely resolved (Fig. 3B). The hematoma volume was 32.87 ml, HUs were 3.36, and midline shift was 22.2 mm, CT performed on POD 177 at the outpatient clinic showed complete resolution of the hematoma (Fig. 3C).
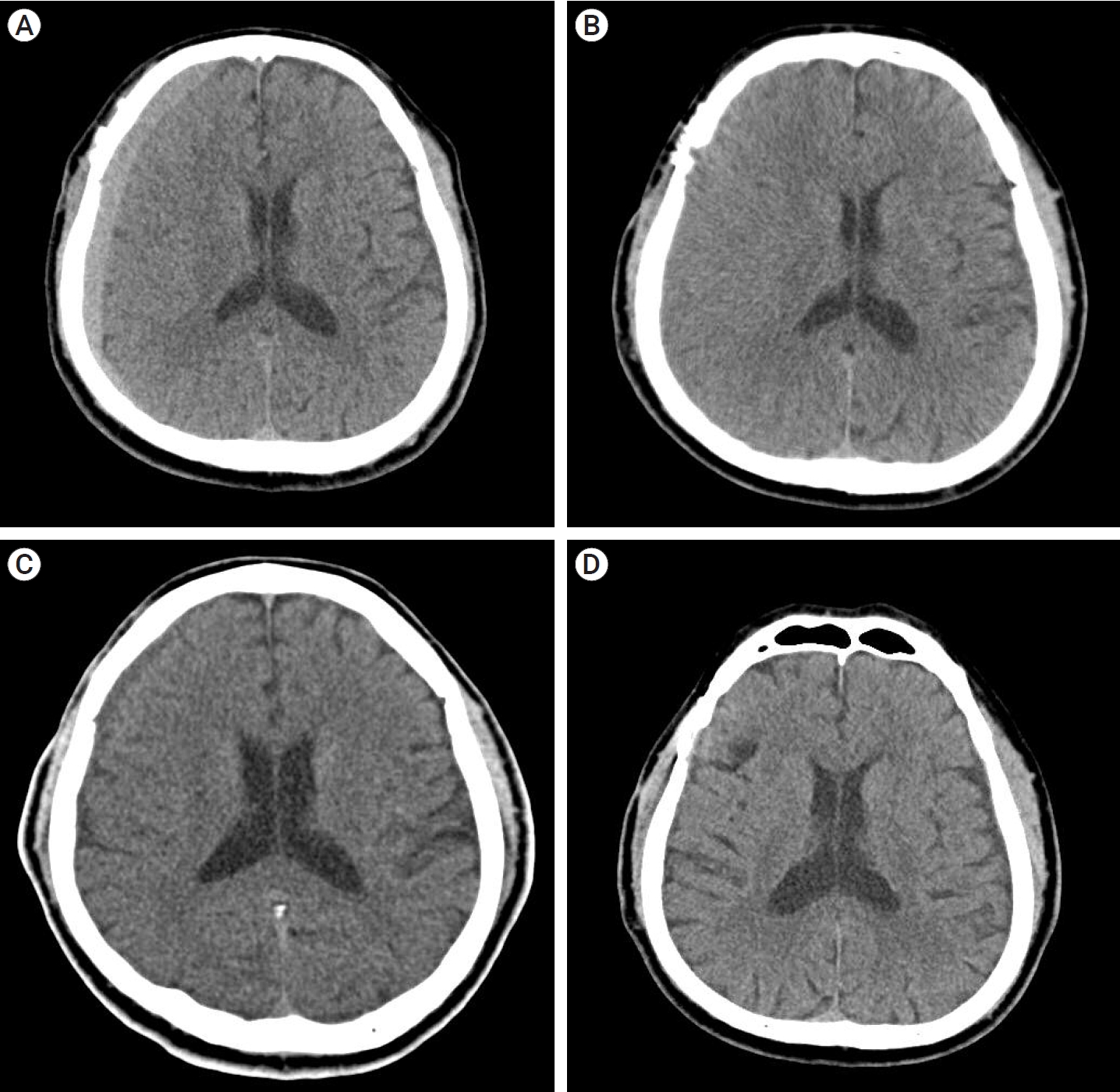
A 68-year-old man was treated with surgical clipping for unruptured right MCA bifurcation and right posterior communicating artery aneurysms. The patient was taking medications for hypertension but was not taking antiplatelet agents. The surgery was performed through ipsilateral pterional approach, and the patient was discharged without any neurologic symptom.
A CT taken on the 42nd POD confirmed high-density CSDH in the frontoparietal area (Fig. 4A); volume was 56.3 ml with a maximum thickness of 9.44 mm, HUs of 59.51, and midline shift of 3.26 mm. A prominent linear low-density area was observed between the hematoma and the brain cortex (Fig. 4B). The patient was observed to be mildly symptomatic (MGS=1) and only had a mild headache (NRS=2), which was easily relieved by acetaminophen. The patient was scheduled for an outpatient visit two weeks later.
A CT scan on the 60th day after surgery showed the hematoma had become iso-dense and decreased in volume compared to the previous scan, and the patientŌĆÖs symptom was also improved. (Fig. 4C); volume was 43.1 ml, HUs was 41.53, maximum thickness was 9.2 mm, and midline shift was 3.23 mm. The third CT scan, taken 112 days after surgery, showed complete resolution of the hematoma (Fig. 4D).
Of our 28 patients, 18 had mild headache symptoms with an MGS of 0-1. The mean maximum hematoma volume was 41.9 ml, and 26 patients had homogeneous type hematoma. Three patients underwent surgery, while 25 showed spontaneous resolution of CR-CSDH. The mean time to hematoma resolution was 126.7 days. Comparing CR-CSDH volume Ōēź43 ml or midline shift Ōēź5 mm, the difference in presence of a linear low-density area and age between the nonoperative and operative groups was found to be statistically significant.
The reported Incidence of CR-CSDH, including cases that do not require surgery, is 2-5% [6,10,16,17]. Several factors, such as advanced age, male sex, and previous brain atrophy have already been identified as unmodifiable predictive factors for CR-CSDH [12]. However, as postoperative subdural fluid collection is considered to be a modifiable predictive factor for CR-CSDH, surgical techniques, such as arachnoidplasty which seals the arachnoid defect, are reported to reduce the incidence of CR-CSDH [8].
Some neurosurgeons have reported spontaneous resolution of CSDH in a case series. Kim et al. suggested conservative follow-up for patients with a CSDH that present with a volume and thickness of <43 ml and 13 mm, respectively, and a midline shift of <5 mm on the head CT scan [7]. Parlato et al. reported that age >70 years, decreased cognitive level, brain atrophy, and absence of an increase in intracranial pressure as signs allowing for conservative treatment [18]. Several nonoperative treatments have also been reported, including steroids, statins, ACE inhibitors, and tranexamic acid [2]. However, as the natural course and optimal management strategy have not yet been established in CR-CSDH, the decision to pursue surgical intervention rests upon the physician with regards to the presence of neurologic symptoms with associated clinical or radiographic evidence of cerebral compression.
Previous reports revealed that approximately 22.2-53.0% of CR-CSDH patients require surgical intervention [6,16,17]. However, in our study, only 10.8% of patients required surgery without conservative management.
The pathophysiology of CSDH remains unclear, but has been hypothesized to be triggered by cleavage of the dural border cell layer after minor trauma, neo-membrane formation and expansion due to coagulopathy, and hyperfibrinolysis with inflammatory cytokines [1]. Focal cerebral contusion and the presence of traumatic subarachnoid hemorrhage (SAH) are also strong predictors of rapid worsening of traumatic CSDH, which echoes other findings [19].
In traumatic CSDH, the arachnoid membrane acts as a sealed door between the pachymeninges and the leptomeningeal space [11]. Eventually, minor arachnoid dissection and CSF accumulation in the dural border cell layer can seed the formation of traumatic CSDH [9,11]. Of course, CR-CSDH can be expanded through a similar mechanism to traumatic CSDH. Our patient with CR-CSDH underwent arachnoid dissection with CSF accumulation in the subdural space during the surgical procedure. Nomura et al. showed that high HU of traumatic CSDH indicates rebleeding and local hyperfibrinolysis, leading to low-density CSDH as time passes [15]. Also, in cases of CR-CSDH, as the HU decrease, the hematoma volume decreases without clinical deterioration over time.
In cases of traumatic subdural hematoma (SDH), clinical progression can be categorized into three periods: the initial period of a traumatic event; the latency period of hematoma expansion; and the clinical period of CSDH manifestation. In the clinical period, as the hematoma manifests, symptoms occur when cerebral compression with hematoma overcomes the patientŌĆÖs ability to compensate [20]. However, despite being produced by a similar mechanism and undergoing similar radiological changes over time, 89.2% of our CR-CSDH patients were asymptomatic or mildly symptomatic based on MGS score, and there was no aggravation during the follow-up period.
We speculate that the reason for the difference in clinical course is that the dissected arachnoid membrane act as a bidirectional door between the pachymeninges and the leptomeningeal space, which causes hemodilution of the CR-CSDH. This pathway induces the dissolution of inflammatory cytokines into the leptomeningeal space and serves as an exit for volume expansion, without clinical aggravation. Therefore, our observations suggest that the natural course and outcome of patients with CR-CSDH strongly depend on the initial pattern of presentation.
Some case examples have reported the radiologic characteristics of spontaneously resolved CSDH. Horikoshi et al. reported radiological findings of spontaneously absorbed CSDH and suggested simultaneous reduction of the hematoma and HU, hematoma in the frontal region, and linear low-density areas as signs of spontaneously absorbed CSDH [4]. This linear low-density area can be interpreted in two ways. The first is that the CSF flow between the hematoma and arachnoid membrane is maintained, and the second is that the hematoma volume is decreasing, which can be considered as a free space between the hematoma and the cortex.
Compared to patients with traumatic CSDH, patients with CR-CSDH undergo wide surgical arachnoid dissection, and the flow between the hematoma and arachnoid membrane is maintained afterwards. In our investigation, the presence of a linear low-density area was found to be statistically significant in the nonoperative group. Because the dissected arachnoid membrane is relatively wide than traumatic CSDH, we assume that there is a high possibility of spontaneous resolution without symptom aggravation.
Naganuma et al. reported four cases of spontaneous resolution of traumatic CSDH and suggested the following three characteristic CT findings associated with spontaneous resolution of traumatic CSDH: low-density hematoma or iso-dense hematoma, small size, and ventricular dilatation [14]. Nomura et al. reported that heterogeneous-type hematomas (layering and mixed type) exhibit a large amount of rebleeding and high coagulative activity [15].
In the case of CR-CSDH, hemodilution of the hematoma can lead to inflammatory cytokines in the CSF, which prevents local hyperfibrinolysis and rebleeding. In our study, 26 patients showed a homogenous-type hematoma during the follow-up period, which resulted in fewer rebleeding events, and the remaining two patients changed to a homogenous pattern within the follow-up period. As the heterogeneous-type of hematoma changes to the homogenous-type over time, the HU also change to low-density without symptom aggravation.
Kim et al. suggested that close observation may be chosen when hematoma volume and thickness are <43 ml and <13 mm, respectively, and midline shift is <5 mm on the CT scan [7]. Here, only 16 CR-CSDH patients (57.1%) fulfilled these criteria. Even if the hematoma exceeded the radiologic criteria, most of the CR-CSDH was spontaneously absorbed. A comparison among CR-CSDH volume Ōēź43 ml or midline shift Ōēź5 mm suggested that age and presence of a linear low-density area on the CT scan were significant in the conservative group. Therefore, we suggest that close observation with serial radiologic examination should be performed if patientŌĆÖs neurologic symptoms are mild and special radiologic finding are present, despite its relatively large volume and midline shifting.
This study has several limitations. First, the study was conducted according to a single protocol at a single center. However, the use of controlled radiologic images and follow-up of chronological findings of CR-CSDH allowed us to have confidence in the results. Second, the incidence of CR-CSDH is relatively low, and the number of operative CR-CSDH cases that we have treated is not large enough; studies with more cases are required to evaluate the clinical course of CR-CSDH. In addition, in the statistical investigation of CR-CSDH production, the number of patients in the comparison and control groups was small, making the statistical analysis difficult. Third, the volume was calculated manually, which is prone to error. Fourth, this study only included patients treated using the pterional approach. The natural course and risk of CR-CSDH may differ among patients treated using other surgical techniques. Fifth, medications reported to be effective in the spontaneous resolution of CR-CSDH were not used in our cases, as their effectiveness was not fully proven. Thus, their mechanisms of action and efficiency have not been thoroughly presented. Sixth, since first follow up CT scan was performed 6-8 weeks after discharge, there may be asymptomatic CR-CSDH which showed complete resolution during this period.
The incidence of CR-CSDH after clipping surgery is not rare. However, CR-CSDH with special radiologic finding does not cause symptom aggravation despite a relatively large volume. The rate of spontaneous resolution or disappearance of the hematoma was within 4 months in most of our cases. Therefore, if the patient with special radiologic finding has mild symptoms other than headache associated with CR-CSDH in the first encounter, close follow-up with serial radiologic examination rather than early surgery may be an attractive option.
Fig.┬Ā1.
Algorithm for the treatment of patients who developed CSDH following surgical clipping. CSDH, chronic subdural hematoma; CT, computed tomography; MGS, markwalder grading scale; NRS, numeric rating scale
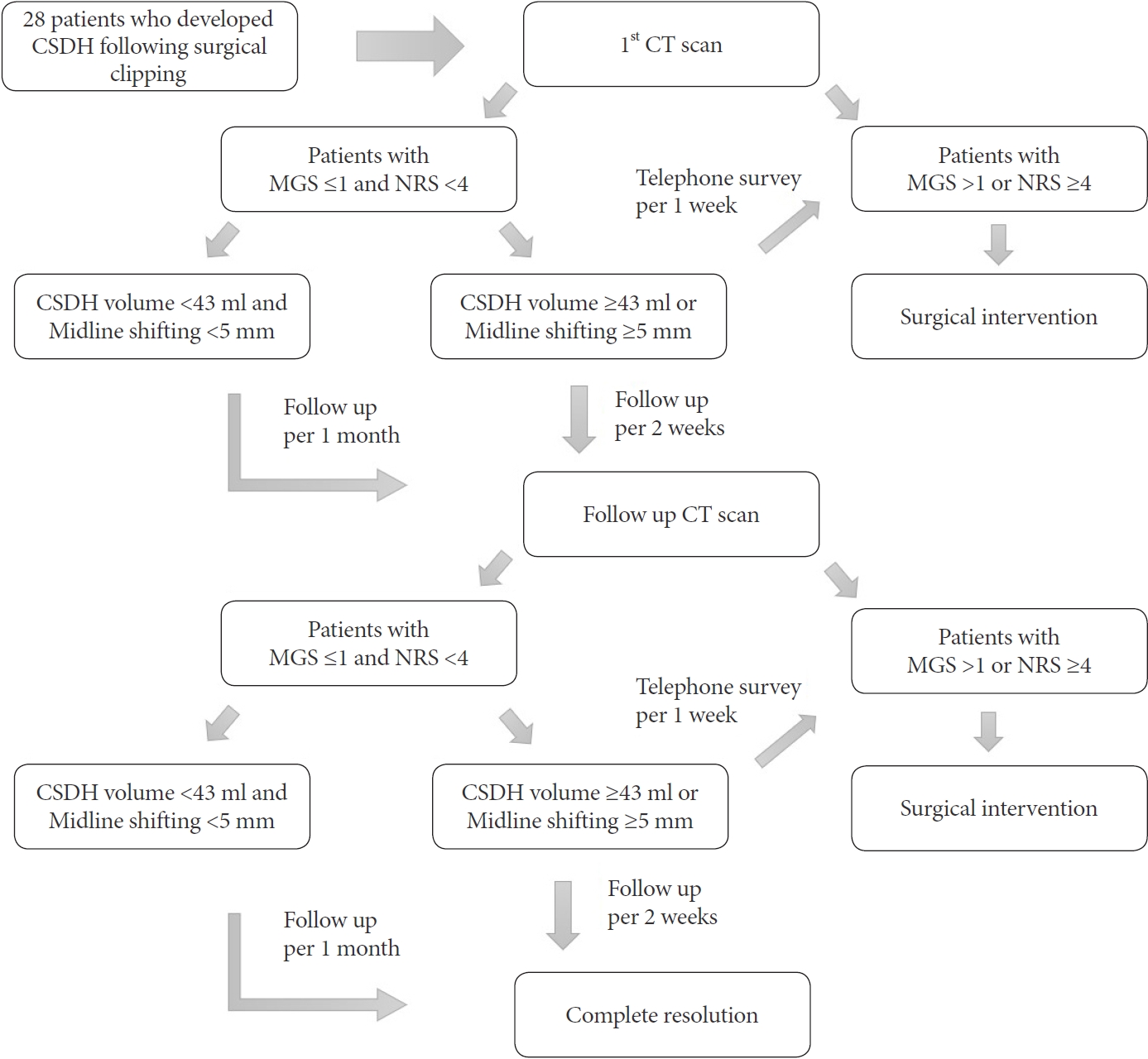
Fig.┬Ā2.
Axial view of CT scan showing CSDH and linear low-density line, with a red arrow pointing to the low-density line which suggesting remained CSF beneath the hematoma. CT, computed tomography; CSDH, chronic subdural hematoma; CSF, cerebrospinal fluid
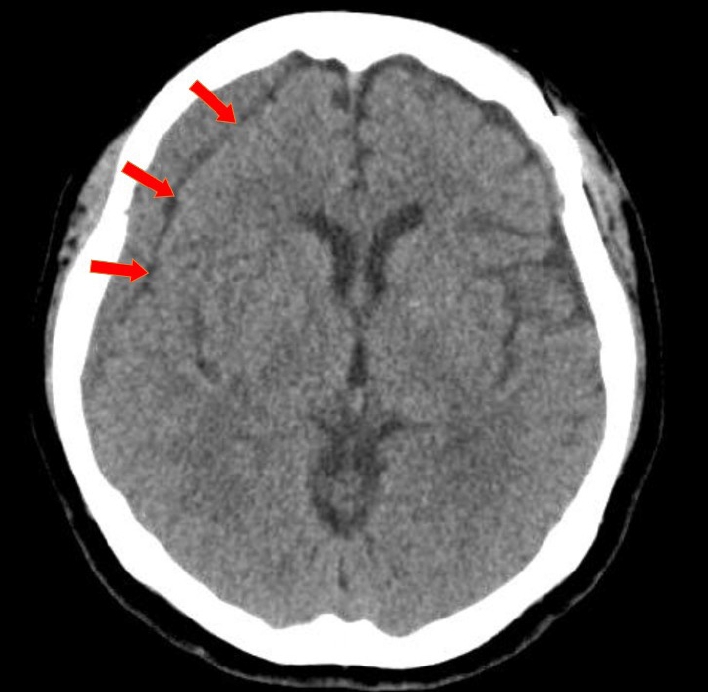
Fig.┬Ā3.
(A) Axial view of CT scan showing a high-density CSDH in the frontoparietal area (Hematoma volume: 78.1 ml, maximum thickness: 17.27 mm, HUs: 58.14, midline shift: 8.34 mm). (B). CT scan of 4th OPD showing CSDH had changed from high-density to hypo-density (Hematoma volume: 32.87 ml, HUs: 3.36, midline shift: 22.2 mm). (C) CT scan of 5th OPD showing complete resolution of CSDH. CT, computed tomography; CSDH, chronic subdural hematoma; HU, hounsfield units; OPD, outpatient department
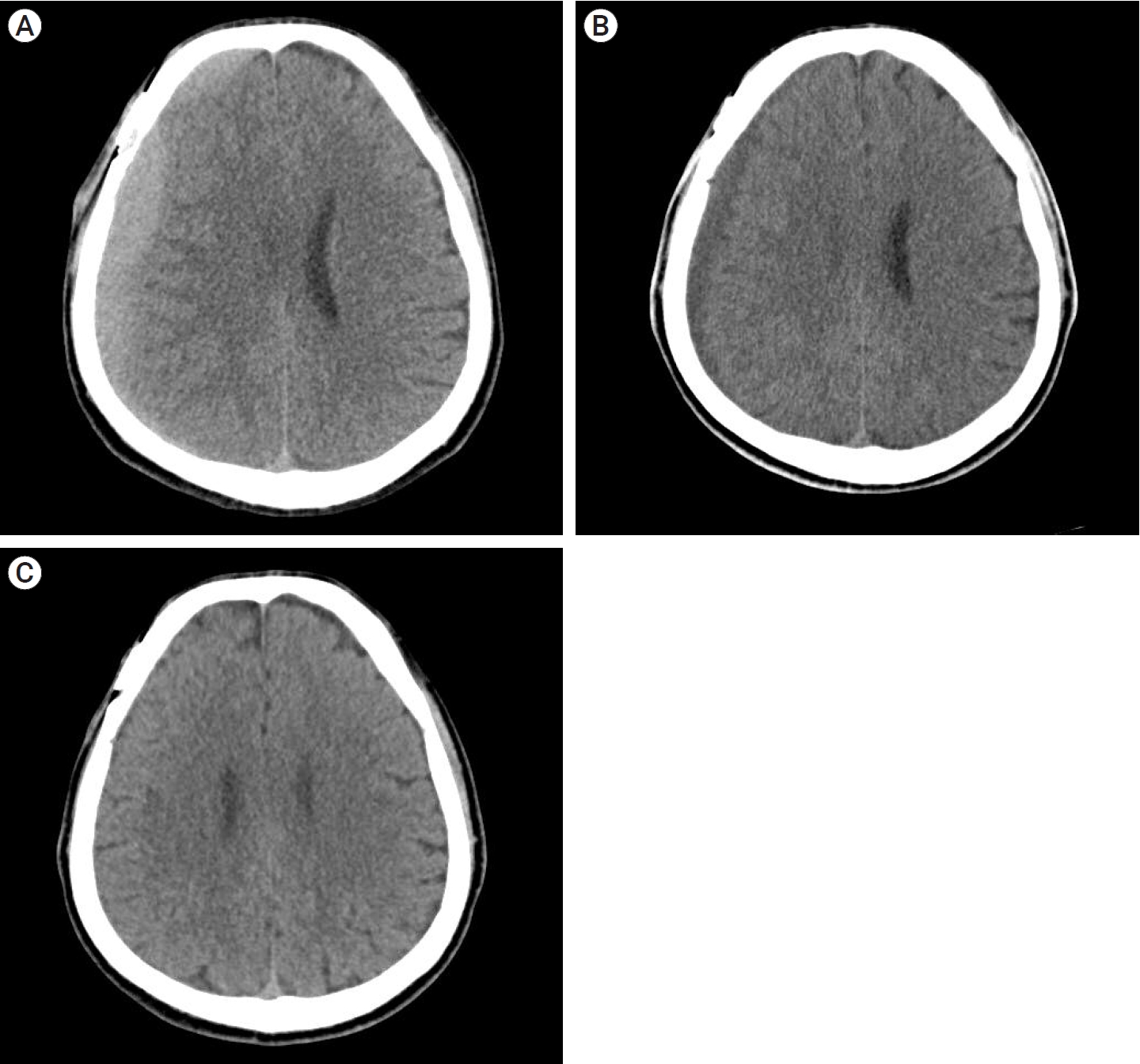
Fig.┬Ā4.
(A) CT scan showing a high-density CSDH in the frontoparietal area (Hematoma volume: 56.3 ml, maximum thickness: 9.44 mm, HUs: 59.51, midline shift: 3.26 mm). (B) CT scan of 2nd OPD showing a decrease in the amount of hematoma, and a linear low-density area between the hematoma and the brain cortex was still observed. (C) CT scan of 3rd OPD showing that the hematoma had become iso-dense (Hematoma volume: 43.1 ml, maximum thickness: 9.2 mm, HUs: 41.53, midline shift: 3.23 mm) (D) CT scan of 4th OPD showing complete resolution of CSDH. CT, computed tomography; CSDH, chronic subdural hematoma; OPD, outpatient department; HU, hounsfield units
Table┬Ā1.
Markwalder grading scale of chronic subdural hematoma
Table┬Ā2.
Baseline characteristics of patients with chronic subdural hematoma after clipping surgery
Table┬Ā3.
Clinical data of patients with radiologic finding and post-operative state after clipping surgery
Table┬Ā4.
Chronological data of nonoperative chronic subdural hematoma after clipping surgery
Table┬Ā5.
Factors affecting surgical intervention of CR-CSDH volume Ōēź43 ml or midline shift Ōēź5 mm
REFERENCES
1. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: Inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. 2017 May;14(1):108.




2. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: Epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. 2020 Sep;141:339-45.


3. Holdgate A, Asha S, Craig J, Thompson J. Comparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute pain. Emerg Med (Fremantle). 2003 Oct-Dec;15(5-6):441-6.


4. Horikoshi T, Naganuma H, Fukasawa I, Uchida M, Nukui H. Computed tomography characteristics suggestive of spontaneous resolution of chronic subdural hematoma. Neurol Med Chir (Tokyo). 1998 Sep;38(9):527-32; discussion 532.


5. Jalbert JJ, Isaacs AJ, Kamel H, Sedrakyan A. Clipping and coiling of unruptured intracranial aneurysms among medicare beneficiaries, 2000 to 2010. Stroke. 2015 Sep;46(9):2452-7.



6. Kang JH, Huh SK, Kim J, Park KY, Chung J. Subdural fluid collection after the clipping of unruptured intracranial aneurysms: Its clinical course and significance. World Neurosurg. 2018 Aug;116:e266-72.


7. Kim HC, Ko JH, Yoo DS, Lee SK. Spontaneous resolution of chronic subdural hematoma: Close observation as a treatment strategy. J Korean Neurosurg Soc. 2016 Nov;59(6):628-36.




8. Kim JH, Kim CH, Lee CY. Efficacy of arachnoid-plasty on chronic subdural hematoma following surgical clipping of unruptured intracranial aneurysms. World Neurosurg. 2017 Aug;104:303-10.


9. Kristof RA, Grimm JM, Stoffel-Wagner B. Cerebrospinal fluid leakage into the subdural space: possible influence on the pathogenesis and recurrence frequency of chronic subdural hematoma and subdural hygroma. J Neurosurg. 2008 Feb;108(2):275-80.


10. Kwon MY, Kim CH, Lee CY. Predicting factors of chronic subdural hematoma following surgical clipping in unruptured and ruptured intracranial aneurysm. J Korean Neurosurg Soc. 2016 Sep;59(5):458-65.




12. Lee WJ, Jo KI, Yeon JY, Hong SC, Kim JS. Incidence and risk factors of chronic subdural hematoma after surgical clipping for unruptured anterior circulation aneurysms. J Korean Neurosurg Soc. 2015 Apr;57(4):271-5.



13. Markwalder TM, Reulen HJ. Influence of neomembranous organisation, cortical expansion and subdural pressure on the post-operative course of chronic subdural haematoma: An analysis of 201 cases. Acta Neurochir (Wien). 1986 79(2-4):100-6.



14. Naganuma H, Fukamachi A, Kawakami M, Misumi S, Nakajima H, Wakao T. Spontaneous resolution of chronic subdural hematomas. Neurosurgery. 1986 Nov;19(5):794-8.


15. Nomura S, Kashiwagi S, Fujisawa H, Ito H, Nakamura K. Characterization of local hyperfibrinolysis in chronic subdural hematomas by SDS-PAGE and immunoblot. J Neurosurg. 1994 Dec;81(6):910-3.


16. Ohno T, Iihara K, Takahashi JC, Nakajima N, Satow T, Hishikawa T, et al. Incidence and risk factors of chronic subdural hematoma after aneurysmal clipping. World Neurosurg. 2013 Nov;80(5):534-7.


17. Park J, Cho JH, Goh DH, Kang DH, Shin IH, Hamm IS. Postoperative subdural hygroma and chronic subdural hematoma after unruptured aneurysm surgery: Age, sex, and aneurysm location as independent risk factors. J Neurosurg. 2016 Feb;124(2):310-7.


18. Parlato C, Guarracino A, Moraci A. Spontaneous resolution of chronic subdural hematoma. Surg Neurol. 2000 Apr;53(4):312-5; discussion 315.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,386 View
- 42 Download
- ORCID iDs
-
Chang-Young Lee

https://orcid.org/0000-0003-2762-1969 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



