Internal carotid artery agenesis presenting with ruptured Acom aneurysm: Rare case report
Article information
Abstract
Developmental anomalies of internal carotid artery (ICA), being rare entities, are mostly asymptomatic by themselves because of good collateral supply. However, when present with other associated intracranial anomalies requiring treatment, there can be catastrophic consequences, if special attention is not paid to this condition. We present a case of 36 years old male, who reported to our emergency department with complaints of headache and loss of consciousness. He was diagnosed as a case of ruptured anterior communicating aneurysm with subarachnoid hemorrhage and agenesis of left ICA with trans-cavernous anastomosis. He underwent clipping of aneurysm and was discharged uneventfully. This report highlights the importance of skillful microsurgical clipping in extremely high-risk conditions, in contemporary era of hybrid neurosurgeons.
INTRODUCTION
Developmental anomalies of internal carotid artery (ICA) are rare entities and can be considered under agenesis, aplasia, and hypoplasia [6]. Most of these cases are asymptomatic in itself [7] because of collateral circulation from the circle of Willis (through the contralateral ICA or vertebrobasilar system) or through the persistent primitive pathways (trans-cavernous anastomosis) or extracranially through the external carotid artery (ECA) [2]. Lie classified these anomalies into six types [6]. When present, symptoms are usually due to the other associated anomalies like intracranial aneurysm, likely resulting from altered hemodynamics and high shear stress. Neurovascular surgeons, particularly hybrid surgeons face dilemma in treating this condition, as difficulty specifically lies in selecting the modality of treatment which can be either endovascular or via microsurgical technique.
CASE DESCRIPTION
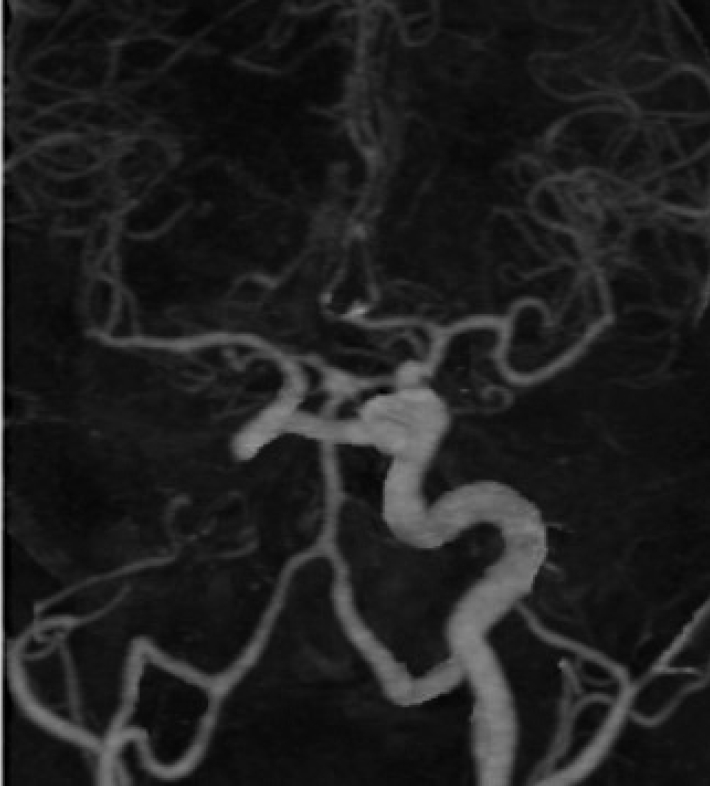
Here is a case of 36 years old hypertensive male, presenting in emergency department with complaints of sudden thunderclap headache, followed by loss of consciousness lasting 10 minutes without any obvious residual deficits. After a thorough clinical assessment, he underwent noncontrast head computed tomography (CT) scan, which was suggestive of interhemispheric and bilateral sylvian subarachnoid hemorrhage (SAH). The patient underwent brain CT angiography, followed by cerebral digital substraction angiography (DSA) which showed a saccular aneurysm arising from right A1-anterior communicating artery (Acom) junction (Fig. 1). There was no filling of left ICA from the origin along with presence of embryonic remnant vessel connecting the cavernous portion of right to left ICA which signifies trans-cavernous pattern of collateralization, solely supplying left middle cerebral artery (MCA) while left anterior cerebral artery (ACA) had supply from contralateral ACA via Acom artery (Fig. 2), which according to Lie’s classification is Type D pattern of collateralization. On detailed review of non-contrast CT scan bony window, there was absent carotid canal on left (Fig. 3). Patient underwent right pterional craniotomy and clipping of aneurysm. Post clipping imaging under indocyanine green (ICG) guidance showed complete obliteration of aneurysm (Fig. 4A) and patent filling of perforators and major vessels (Fig. 4B). Post operative period was unremarkable and he was discharged uneventfully on 7th post operative day with modified Ranking score of 0.
CT angiogram (head) displaying left ICA agenesis, inter-cavernous anastomosis from right to left ICA, left distal ACA supplied by anterior communicating artery (Acom) via right A1 segment. Small outpouching (aneurysm) can be seen arising from Acom segment. CT, computed tomography; ICA, internal carotid artery; ACA, anterior cerebral artery
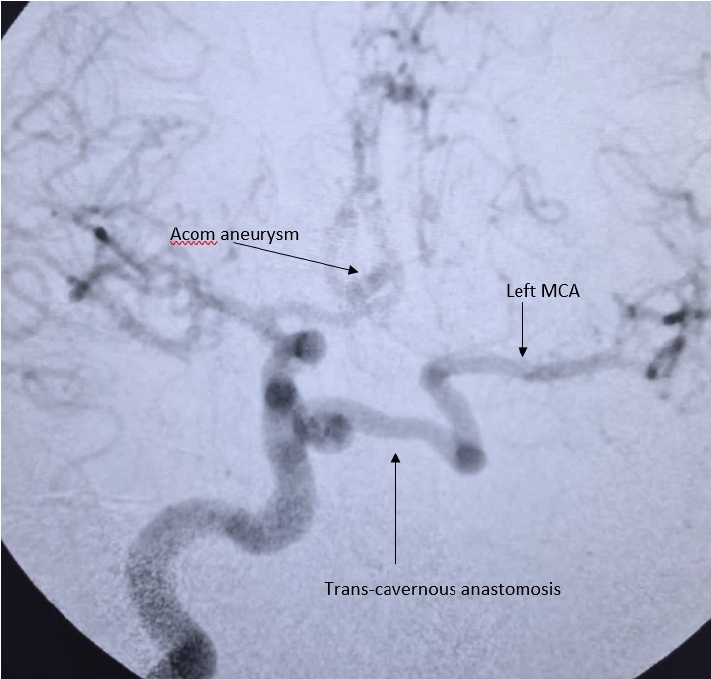
Selective right ICA DSA shows saccular aneurysm arising from Acom segment. Trans-cavernous anastomosis can be seen arising from right ICA, which is the sole supply for left MCA. Left distal ACA is supplied by patent Acom artery. ICA, internal carotid artery; DSA, digital substraction angiography; Acom, anterior communicating artery; MCA, middle cerebral artery; ACA, anterior cerebral artery

Noncontrast head CT bony window where darker arrow denotes absent carotid canal on left which has been replaced by pneumatization, lighter arrow denotes patent right carotid canal. CT, computed tomography

(A) Intra-operative microscopic view of clipped A1-Acom junction aneurysm (blue arrow – right A1, black arrow – recurrent artery of Heubner, arrow head – right A2, green arrow – left A1, cross – Acom) (2 clips were applied for complete aneurysm occlusion). (B) Intra-operative microscopic view under ICG guidance where complete occlusion of aneurysm is noted with patent major and perforating vessels. Acom, anterior communicating artery; ICG, indocyanine green
DISCUSSION
This condition was first described incidentally on postmortem examination by Tode [7] in 1787 and was demonstrated on angiography by Verbiest [3]. Although ICA agenesis is rare, with the reported incidence of ICA development anomalies being less than 0.01% [5], its knowledge to hybrid neurovascular surgeon is imperative, as its treatment can be challenging. It occurs almost equally among male and females with common age at presentation being 5th and 6th decade and anomalies on the left side is more common than the right [1].
Lie classified ICA agenesis into six different categories [6], Type A, the ACA of the affected side is supplied by the contralateral ICA through the contralateral A1 via Acom, while the MCA of the affected side is supplied by the posterior circulation through ipsilateral posterior communicating artery (Pcom). In type B, both the ACA and MCA of the affected side are supplied by the contralateral ICA through the contralateral A1 and Acom. Type D describes a trans-cavernous or inter-cavernous pattern of supply, in which an embryonic vessel remnant originating from the cavernous portion of the normal ICA persists. This remnant vessel reconstitutes blood flow either by anastomosing with the cavernous portion of the affected ICA or by directly supplying the MCA of the affected side. Type C & E represent bilateral ICA agenesis while Type F characterizes unilateral or bilateral “rete mirabile” a web-like mesh of thin arteries at the skull base connecting and supplying the anomalous ICA through the ECA [6].
As compared to incidence of aneurysm which is 2-4% in general population, incidence of aneurysm among individuals with ICA agenesis has been reported around 29% [4]. Acom aneurysm is the most common aneurysm reported among ICA agenesis cases [10]. Most patients are asymptomatic due to the collateral blood supply from either circle of willis or ECA, nevertheless some patients can present with headache, seizure, and neurological deficit due to ischemia and can also present with SAH or parenchymal Hemorrhage.
Diagnosis of ICA agenesis can be made by DSA, CT angiography (CTA), magnetic resonance angiography (MRA) and by the evidence of absent carotid canal on CT. Our patient presenting aneurysmal SAH with ICA agenesis was diagnosed on CTA and confirmed on DSA. The carotid canals should always be evaluated during the reading of routine cranial CT studies with axial slices and bone window images, considering that this is the confirmatory sign of the ICA absence [9], not adding to acquisition time or cost of study.
According to Lasjaunias & Vazquez [8], the ICA is formed by the seven distinct segments. Each segment is independent and may show agenesis. Agenesis of one segment leads to regression of all segments proximal to the anomaly. In our case, there was agenesis of the first segment resulting in the filling from the contralateral ICA via the primitive trans-cavernous anastomosis.
Treatment of aneurysms in such cases poses a dilemma to the neurosurgeon in selecting modality, which can be either endovascular or microsurgical. Either of these treatment modalities has its own merits and demerits. During clipping there are chances of damage to single ipsilateral ICA when temporary clip is applied and while dissection of aneurysm, injury to important perforators can occur, leading to deficits. We avoided applying temporary clip to ICA, keeping this anomaly in mind and opted for clipping as it completely obliterates the aneurysm, and the patient was young with good clinical condition. Endovascular coiling can also cause complications like thromboembolic events, spasms etc of the single ipsilateral ICA and its branches which can have grave consequences. Moreover, patients of endovascular coiling require regular radiographic follow-up to look for regrowth or complete obliteration, making patient compliance mandatory, which can be a limiting factor. Detailed patient counseling should be done before commencing any treatment and should preferably be performed in institutes where neurovascular cases are routinely performed.
Important implications are there if this anomaly is present for other cases, like failure to recognize the intercavernous collateral can result in grave consequences during transsphenoidal surgery for pituitary macroadenoma and while performing carotid endarterectomy (CEA) for carotid stenosis.
CONCLUSIONS
Although ICA agenesis is a rare congenital development anomaly of the ICA and itself is rarely symptomatic but can cause manifestations as and when associated with other anomalies like intracranial aneurysms or other cerebrovascular abnormalities, so every neurosurgeon must have knowledge of this condition in their diagnostic armamentarium, along with merits and demerits of available treatment options, as it can pose a difficult predicament while choosing a modality, either endovascular or microsurgical clipping as in cases of aneurysms.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
