 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 16(3); 2014 > Article |
|
Abstract
Objective
The objective of this study is to evaluate the clinical and angiographic outcomes after primary balloon angioplasty in patients with symptomatic middle cerebral artery (MCA, M1 segment) stenosis refractory to medical therapy.
Materials and Methods
Eleven patients with intracranial stenosis were treated with primary balloon angioplasty. All patients had MCA stenosis with recurrent transient ischemic attack (TIA). The indication for balloon angioplasty was patients with significant MCA stenosis: 1) age older than 18 years with recurrent or progressive TIA or infarction despite optimal medical therapy, including anti-coagulation, dual anti-platelet, and anti-lipid medication; 2) previous ischemic events or asymptomatic severe stenosis (more than 50%) with poor collateral cerebral circulation, or diminished cerebral perfusion on single photon emission computed tomography before and after administration of the intravenous dosage of acetazolamide.
Results
The median age of patients was 53 years (range 44-79). The technical success rate was 100%. Mean pretreatment stenosis degree was 83.63 ┬▒ 9.53% and 29.1 ┬▒ 15.4% before and after angioplasty, respectively. Procedural-related complications occurred in four of 11 patients (36%), but none of the patients had permanent neurological deficit. All patients were available for an average follow-up period of 19.4 ┬▒ 5.1 months. One patient had a stroke in the territory of angioplasty at two months after angioplasty. The stroke free survival rate at 30 days and 12 months was 100% and 91%, respectively. Restenosis over 50% was observed in three of 11 patients (27%); all were asymptomatic.
The best treatment for middle cerebral artery (MCA) stenosis remains controversial. Outcomes of medical treatment for patients with MCA stenosis are unfavorable. Reported annual stroke rates are 8%-10%.22) The warfarin-aspirin symptomatic intracranial disease study reported that medical treatment with warfarin or aspirin resulted in failure to prevent further ischemic events.4) The 1-year 2-year probability for recurrent stroke in the territory of the stenotic artery in patients treated with aspirin was reported as 12% and 15%, respectively.4)16)
Recently, in patients with intracranial arterial stenosis, aggressive medical management (SAMPRIS study) was reported to be superior to primary balloon angioplasty with the use of the Wingspan stent system.4) However, primary balloon angioplasty was found to be effective for selected patients of symptomatic intracranial stenosis. Technically, balloon angioplasty is a simple technique compared to stenting. Considering tortuous cerebral arteries, easy accessibility is one of the prominent benefits of balloon angioplasty. Balloon angioplasty has several shortcomings, including the possible risk of intimal elastic recoil, dissection, and residual stenosis because of the use of small caliber balloons.5)13) However, for small sized cerebral arteries, MCA in particular, efficacy and safety of primary stenting is still contentious. In our practice, medial management has been the first option, with only select patients undergoing endovascular treatment. These patients include symptomatic severe MCA stenosis (over 50%) with recurrent symptoms despite adequate medical treatment. In this study, we summarize the clinical and radiological results of primary balloon angioplasty for MCA stenosis.
We retrospectively assessed 11 consecutive patients with symptomatic MCA stenosis who underwent primary balloon angioplasty at our institution between Jan 2007 and Dec 2009. The patients comprised eight men and three women with a median age of 53 years (range, 44-79 years). All patients had provided written informed consent for the procedure.
All patients had symptomatic intracranial stenosis. Most patients had recurrent transient ischemic attack (TIA) or stroke despite receiving antiplatelet and antilipid medication. The clinical and angiographical parameters evaluated were patient age and sex, clinical presentation, degree of stenosis before and after balloon angioplasty, antiplatelet and anticoagulation regimen, procedure-related complications, and clinical and angiographic consequences at follow-up.
Symptomatic MCA stenosis was defined as the occurrence of one or more TIA or non-disabling strokes in the MCA territory within six months. The indication of balloon angioplasty was patients with significant MCA stenosis in these situations: 1) age older than 18 years with recurrent or progressive TIA or infarction despite optimal medical therapy, including anti-coagulation, dual anti-platelet, and anti-lipid medication; 2) previous ischemic events or asymptomatic severe stenosis (more than 50%) with poor collateral cerebral circulation, or diminished cerebral perfusion on technetium-99m ethyl cysteinate dimer (ECD) single photon emission computed tomography (SPECT) before and after administration of the intravenous dosage of acetazolamide 1 g (Zoladin, Far-East Pharmaceuticals, Seoul, Korea).
Exclusion criteria were: 1) stenosis distal to the MCA bifurcation, 2) severe neurologic deficits (mRS Ōēź 4) in the influenced MCA territory, 3) life expectancy is short, presumably < 5 years, 4) cardiac lesions likely to cause cardiac thromboembolism, 5) chronic complete occlusion of the MCA, 6) patients with tandem intracranial and extracranial disease undergoing angioplasty at both sites. Therefore, this study did not include patients with hemodynamically significant extracranial cerebrovascular disease at the time of angioplasty.
The degree of MCA stenosis (S) expressed as a percentage was measured by comparing the diameter of the vessel at the site of highest stenosis (Dsten) with the normal diameter of the vessel just distal to the stenosis (Ddist), as follows: S[1(Dsten/Ddist)] ├Ś 100 using the WASID method. Overall vessel sizing was done with catheter angiograms including three-dimensional reconstructed images. The definition of restenosis means displayed stenosis over 50% at the time of follow-up.
The patients received 100 mg of aspirin and 75 mg clopidogrel daily for at least seven days before the procedure. A bolus of 3000-5000 U of heparin was administered intravenously at the beginning of the procedure. An additional 1000 IU bolus of heparin was administered every hour. All endovascular procedures were performed under general anesthesia. Entire diagnostic cerebral angiography was performed in each patient. The stenotic segment of the MCA was contacted using a 0.014-inch coronary microwire, which was navigated into the insular portion of the MCA for maximal support; this allowed tracking of the coronary balloon catheter (Mercury, Abbott, Abbott Park, IL, United States / Sprinter, Medtronic, Minneapolis, MN, United States). Patients were treated with semi-compliant coronary angioplasty balloons varying in size from 1.5 ├Ś 9 mm to 2.5 ├Ś 20 mm inserted through 5 F to 7 F guide catheters. Balloons were deliberately undersized by 80% of normal diameter in order to decrease the risk of arterial damage or rupture using angiographically obtained measurements. The available length to fully cover the lesion was chosen and balloons were slowly inflated for three minutes. After the procedure, patients were generally continued on dual antiplatelet medication for two years. No anticoagulation was administered after the procedure (Figs. 1, 2).
Pretreatment stenosis varied from 60-95% (mean 83.63 ┬▒ 9.53%). Ten (91%) of the lesions had more than 70% stenosis, with only one patient (9%) having stenosis in the 50-69% range. All lesions were crossed and dilated successfully. Post-treatment stenosis varied between 5% and 60% (mean 29.1 ┬▒ 15.37%). Eight patients (72%) had less than 50% residual stenosis, three patients (28%) had 50-69% stenosis (Table 1).
Procedural events occurred in four (36.4%) of 11 patients, but no patient had permanent neurological deficit. One patient developed acute thromboembolism during the procedure, but showed improvement after treatment with thrombolysis (urokinase 200,000 IU and tirofiban hydrochloride 300 mg). Three patients developed dissection of MCA. If dissection was seen, we observed it for 10-30 minutes. None of the dissections produced flow stagnation, embolism, progression, or ischemic symptoms.
There were no complications attributable to vessel rupture, reperfusion hemorrhage, and branch occlusion associated with an ischemic infarct.
There were no deaths or strokes within 30 days of the procedure. All patients were available for follow-up, which varied from 2-41 months (mean 19.36 months). During follow-up, one patient experienced a recurrent stroke in the vascular distribution of the treated vessel. Routine follow-up imaging with conventional cerebral angiography at one year, computed tomographic angiography or magnetic resonance angiography at three or six months was performed on all patients. Four patients underwent conventional angiography, four patients underwent computed tomographic angiography, and three patients underwent magnetic angiography. Three of 11 patients (27%) had asymptomatic restenosis over 50%, but did not require repeated angioplasty.
Application of coronary balloon-expandable bare metal stents in cerebral arteries has been reported with favorable results. However, in real practice, we have experienced various complications associated with intracranial coronary stents, particularly in cases of M1 stenosis, which included proximal or distal MCA branches rupture-perforation, perforator occlusion, early and delayed thromboembolism, and frequent restenosis.6)12)20) All have been real concerns in our practice. Many aspects of endovascular strategies for intracranial stenosis have come from the coronary field; however, the cerebral artery is different from the coronary vessels in several aspects. First, the vascular structure and environment of arteries are obviously different from those of coronary arteries.17) The differences include the absence of an external elastic lamina, lack of adventitia, thin media in the arterial wall, and suspension in cerebrospinal fluid. All represent weak arterial walls of cerebral vessels.
The mechanisms of stroke are also different. There are three possible mechanisms of cerebral stroke; thromboembolism, perforator occlusion, and hypoperfusion.7) Each patient can have one or combined etiologies. Pathophysiologic aspects should be considered for individual patients and lesions. For example, when perforator occlusion is the main mechanism, endovascular treatment of the stenosis may add unnecessary risks. Benefit would be rare. If hypoperfusion matters, simple dilatation may lead to dramatic improvement. According to the Hagen-Poiseuille equation, double the radius of the vessel results in a 4th power increase of the blood volume. Therefore, just a 10% increase of the diameter will lead to double the increase of the current flow volume. This implies that returning to the normal diameter of the stenotic arteries may not be essential to resolving a perfusion issue. Suboptimal dilatation of the vessel would be sufficient in cases of hypoperfusion. Again, we could achieve clinical benefit even with less aggressive dilatation of the stenosis.
Almost all patients with symptomatic intracranial stenosis are symptomatic due to thromboembolic factors.3)7) In cases of thromboembolism, we should consider that the plaque could be unstable. In the early period after symptom occurrence, it is more vulnerable. In addition, the cerebral artery has a somewhat different plaque nature compared to the traditional concept of unstable plaque of coronary arteries. In cerebral arteries, percent stenosis is the highest in cured plaque rupture and is greater than that of thin-cap atheromas and acute plaque ruptures.
For cervical carotid stenosis, early surgery has been the rule. In recent practice, carotid stenting has also been performed during the acute period in order to reduce further occurrence of stroke, accounting for increased risk of procedure-related thromboembolism. However, there are various protection devices. There is no protection device for cerebral circulation. Therefore, a less aggressive approach to cases of stroke due to thromboembolism from the intracranial stenosis is needed. Thromboembolic complication risk of endovascular stenting is 16-56.3% and that of balloon angioplasty is 2%.11)15)
An attempt at stent deployment may not be appropriate in certain circumstances. In cases where tortuous anatomy would preclude or potentially complicate stent placement, where the stenosed arteries to be treated are too small in diameter or too distal to be stented, or where the stenosed arterial segment is longer than any available stent of the appropriate diameter, balloon angioplasty alone may be the only desired dilation step. The safety and device performance of a new family of self-expanding stents specifically designed for treatment of atherosclerotic lesions was assessed.2) The nitinol stent (Wingspan, Boston Scientific/Smart, Fremont, CA, United States) has two times greater radial force than the Neuroform stent, and is suitable for distal internal carotid artery and MCA lesions that are too rigid to access or are not accessible using a balloon-mounted stent. The safety of these procedures may be compromised by limited flexibility of the coronary balloons or balloon-mounted stent delivery systems, high inflation pressure required for deployment of stainless steel stents in fragile intracranial vessels, risk of shearing the stent from the balloon while navigating to the objective lesion, and difficulty in accurately sizing the balloons and stents according to the vessel diameter.
Initial case series reported relatively high rates of periprocedural morbidity using the angioplasty-alone strategy.5) The introduction of routine adjunctive periprocedural platelet inhibition, along with a more conservative approach to balloon sizing and a slow balloon inflation technique, made intracranial angioplasty considerably safer. Several single institution retrospective series and a multicenter retrospective series have suggested that with these modern techniques intracranial angioplasty without stenting can be performed with a relatively low periprocedural risk profile.15)16) The major potential limitations of angioplasty without stenting include vessel recoil with acute recurrent stenosis, acute vessel occlusion secondary to either procedural dissection or recoil with local platelet aggregation, and a lack of long-term durability, with some patients requiring multiple procedures because of recurrent stenosis. In an additional 12.9% of patients treated, stents were required because the initial angioplasty result was unchanged or worse than the pretreatment stenosis. In the current series, only four of 81 (4.9%) lesions treated with both the Gateway balloon and Wingspan stent remained with Ōēź 50% stenosis at the conclusion of the initial procedure. Long-term follow-up is ongoing.2)9)23)
In patients with intracranial arterial stenosis, aggressive medical management was superior to primary balloon angioplasty with the use of the Wingspan stent system, both because the risk of early stroke after primary balloon angioplasty was high and because the risk of stroke with aggressive medical therapy alone was lower than expected (SAMPRIS study).4) However, in some studies, primary balloon angioplasty was effective for symptomatic intracranial stenosis with risk of stroke or death.16)20) Intracranial angioplasty without stent placement has not been studied prospectively. Technical success (defined as reduction of stenosis to < 50%) can be achieved in > 80% of patients and the rate of stroke or death within 30 days of angioplasty varied between 4% and 40% in several retrospective angioplasty studies.5)13) Various elective procedures have been associated with lower complication rates (4-6%). Restenosis rates following angioplasty alone range from 24-40%.7) Marks et al. recently reported an average follow-up period of 52.9 months in 36 patients showing an annual stroke rate of 3.36% in the territory proper to the site of angioplasty.15)
Important drawbacks in balloon angioplasty include intimal dissection, early elastic arterial recoil, and immediate high-grade residual stenosis.10)13)18)20)21) To overcome these drawbacks, use of undersized balloons and slow balloon inflation is recommended, but residual stenosis > 50% and dissection are still reported in 16% and 14%,13)5) respectively.
Even though an excellent outcome was achieved for balloon dilation in a single center study without recoil, and with long-term stability compared with stenting, this study, which was conducted with a relatively small number of cases (11 patients) and with no control group, is not sufficient to definitively conclude the feasibility and safety of balloon angioplasty for MCA stenosis treatment. We cannot rule out the presence of unknown, confounding variables not accounted for in the final analysis. If the operator could depend on balloon angioplasty alone, it would be an ideal, usually simple to perform, procedure with minimal risk; there would be no need for stent placement, with all the associated possible problems related to placement of a permanent metallic foreign body in the artery, from the actual deployment process through late restenosis or thrombosis and the requirement for prolonged dual antiplatelet therapy. Further clinical trials with a large, well-designed, randomized, controlled trial are required in order to confirm our results.
We found a high rate of technical success and a low rate of stroke recurrence and residual stenosis, suggesting that this procedure has a durable effect. From this result, in treatment of symptomatic MCA stenosis refractory to medical treatment, we found that only primary balloon angioplasty without aggressive stent placement is a preferable and alternative treatment modality.
Adoption of a practice based on comparative data derived from coronary interventions suffers from a lack of understanding of the differences between interventions in the two vascular beds. Currently available endovascular devices for dilating MCA stenosis are not yet perfect, and are associated with significant risk. All of the above indicate that a conservative policy is needed for cases of small intracranial arteries like MCA. If endovascular treatment should be chosen, we believe that less aggressive procedures should be the first option. The outcomes highlight the need for conduct of randomized clinical trials with patient selection, perioperative medical management, and a highly skilled neuroendovascular surgeon in order to determine the precise value of balloon angioplasty and stenting in intracranial MCA stenosis.
References
1. Abou-Chebl A, Bashir Q, Yadav JS. Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke. 2005 12;36(12):e165-e168;


2. Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007 5;38(5):1531-1537;


3. Carlsson J, von Wagenheim B, Linder R, Anwari TM, Qvist J, Petersson I, et al. Is late stent thrombosis in drug-eluting stents a real clinical issue? A single-center experience and review of the literature. Clin Res Cardiol. 2007 2;96(2):86-93;



4. Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Warfarin-aspirin symptomatic intracranial disease trial investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005 3;352(13):1305-1316;


5. Connors JJ 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg. 1999 9;91(3):415-423;


6. Coward LJ, McCabe DJ, Ederle J, Featherstone RL, Clifton A, Brown MM. CAVATAS Investigators. Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke. 2007 5;38(5):1526-1530;


7. Derdeyn CP, Chimowitz MI. Angioplasty and stenting for atherosclerotic intracranial stenosis: rationale for a randomized clinical trial. Neuroimaging Clin N Am. 2007 8;17(3):355-363;



8. Fiorella D, Chow MM, Anderson M, Woo H, Rasmussen PA, Masaryk TJ. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007 8;61(2):236-242; discussion 242-3.



9. Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007 3;38(3):881-887;


10. Gress DR, Smith WS, Dowd CF, Van Halbach V, Finley RJ, Higashida RT. Angioplasty for intracranial symptomatic vertebrobasilar ischemia. Neurosurgery. 2002 7;51(1):23-27; discussion 27-9.



11. Kim JK, Ahn JY, Lee BH, Chung YS, Chung SS, Kim OJ, et al. Elective stenting for symptomatic middle cerebral artery stenosis presenting as transient ischaemic deficits or stroke attacks: short term arteriographical and clinical outcome. J Neurol Neurosurg Psychiatry. 2004 6;75(6):847-851;



12. Lee CY, Yim MB. Primary stent therapy for symptomatic intracranial atherosclerotic stenosis: 1-year follow-up angiographic and midterm clinical outcomes. J Neurosurg. 2006 8;105(2):235-241.

13. Lee JH, Kwon SU, Lee JH, Suh DC, Kim JS. Percutaneous transluminal angioplasty for symptomatic middle cerebral artery stenosis: long-term follow-up. Cerebrovasc Dis. 2003 15(1-2):90-97;


14. Lutsep HL. Symptomatic intracranial stenosis: best medical treatment vs. intracranial stenting. Curr Opin Neurol. 2009 2;22(1):69-74;


15. Marks MP, Marcellus ML, Do HM, Schraedley-Desmond PK, Steinberg GK, Tong DC, et al. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow-up. AJNR Am J Neuroradiol. 2005 3;26(3):525-530;


16. Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006 4;37(4):1016-1020;


17. Miao ZR, Feng L, Li S, Zhu F, Ji X, Jiao L, et al. Treatment of symptomatic middle cerebral artery stenosis with balloon-mounted stents: long-term follow-up at a single center. Neurosurgery. 2009 1;64(1):79-84; discussion 84-5.



18. Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998 9;19(8):1525-1533;




19. Puetz V, Gahn G, Becker U, Mucha D, Mueller A, Weir NU, et al. Endovascular therapy of symptomatic intracranial stenosis in patients with impaired regional cerebral blood flow or failure of medical therapy. AJNR Am J Neuroradiol. 2008 2;29(2):273-280;



20. Qureshi AI, Hussein HM, El-Gengaihy A, Abdelmoula M, K Suri MF. Concurrent comparison of outcomes of primary angioplasty and of stent placement in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 2008 5;62(5):1053-1060; discussion 1060-2.


21. Suh DC, Kim JK, Choi JW, Choi BS, Pyun HW, Choi YJ, et al. Intracranial stenting of severe symptomatic intracranial stenosis: results of 100 consecutive patients. AJNR Am J Neuroradiol. 2008 4;29(4):781-785;



Fig.┬Ā1
A 56-year-old woman had recurrent transient ischemic attack (TIA) symptoms refractory to medical treatment. (A) Anteroposterior left internal carotid artery (ICA) angiogram shows severe stenosis (> 50%) in the proximal M1 portion of the left middle cerebral artery (MCA). (B) Angioplasty balloon is performed successfully. (C) Postprocedural ICA angiogram shows the MCA with a smooth appearance, widened or increased luminal diameter (residual stenosis about 30%), and preservation of the lenticulostriate arteries. (D) After angioplasty, the patient developed weakness of the upper extremity. Anteroposterior left ICA angiogram shows stagnation of distal MCA cortical flow, possibly due to multiple distal emboli. (E) After chemical thrombolysis (urokinase 200,000 IU and tirofiban Hydrochloride 300 ┬Ąg), weakness of upper extremity shows improvement (grade 5). (F) Angiogram 15 months after the procedure does not show significant re-stenosis.
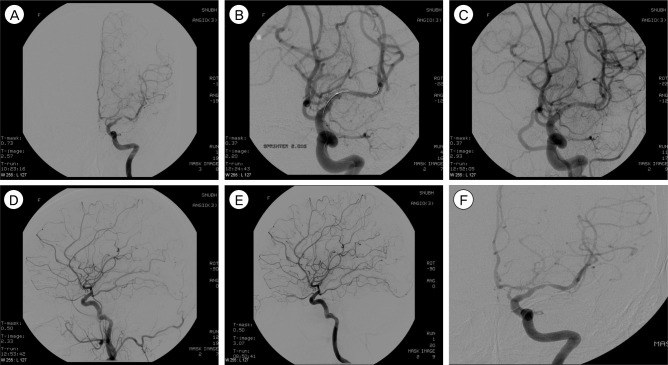
Fig.┬Ā2
A 53-year-old man had recurrent TIA symptoms refractory to medical treatment. (A) Anteroposterior left ICA angiogram shows severe stenosis (approximately 90%) in the proximal M1 portion of the left MCA. (B) Angioplasty balloon was deployed successfully. During angioplasty, an angiogram demonstrates dissection of MCA. However, the patient did not show any neurological deficit. (C) Postprocedural ICA angiogram (delayed 30 minutes) shows the MCA with a smooth appearance, widened luminal diameter (residual stenosis about 50%), and preservation of the lenticulostriate arteries. (D) Magnetic resonance angiogram at 23 months after the procedure shows restenosis (approximately 60%). However, the patient had no ischemic event.

Table┬Ā1
Summary of clinical and angiographic characteristics of patients treated with balloon angioplasty in intracranial MCA stenosis (N = 11)

MCA = middle cerebral artery; M = male; F = female; TIA = transient ischemic attack; DM = diabetes mellitus; HT = hypertension; F/U = follow up; CTA = computed tomographic angiography; TFCA = transfemoral carotid angiography; MRA = magnetic resonance angiography; PMHx = past medical history; GOS = Glasgow outcome scale.
- TOOLS
-
METRICS

-
- 6 Crossref
- 0 Scopus
- 2,417 View
- 26 Download
- Related articles
-
Microsurgical Anatomy of the Middle Cerebral Artery.2000 September;2(2)
Stenting of Symptomatic Middle Cerebral Artery Stenosis: Case Report.2002 September;4(2)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



