Geometric influence of anterior cerebral artery rotation on the formation of anterior communicating artery aneurysm
Article information
Abstract
Objective
Several particular morphological factors that contribute to the hemodynamics of the anterior communicating artery (ACoA) have been documented, but no study has investigated the role of the degree of anterior cerebral artery (ACA) rotation on the presence of ACoA aneurysms (ACoAAs).
Methods
A retrospective study of an institutional aneurysm database was performed; patients with ruptured or nonruptured ACoAAs were selected. Two sex- and age-matched control groups were identified: control Group A (nonaneurysms) and control Group B (middle cerebral artery aneurysms). Measurements of ACA rotation degree were obtained by using a three-dimensional imaging tool.
Results
From 2015 to 2020, 315 patients were identified: 105 in the ACoAA group, 105 in control Group A, and 105 in control Group B. The average age at the time of presentation was 64 years, and 52.4% were female. The ACA rotation degree of the ACoAA group was significantly higher than that of control Group A (p <0.01). The A1 ratio and the A1A2 ratio of the ACoAA group were greater than those of control Group A (p <0.01 and p <0.01, respectively). The ACA rotation degree correlated insignificantly with aneurysm size in ACoAA patients (p=0.78). The ACA rotation degree in the ACoAA group was also insignificantly different from that in control B (p=0.11).
Conclusions
The degree of ACA rotation was greater in the ACoAA group than in the nonaneurysm group, and it may serve as an imaging marker for ACoAA.
INTRODUCTION
A cerebral aneurysm (CA) is an outpouching of a weakened arterial wall, represents an abnormal dilation on the cerebral arterial wall, and leads to nontraumatic subarachnoid hemorrhage (SAH), accounting for approximately 75-85% of spontaneous SAHs [10,16,22,28,31]. The reported risk of small anterior circulation of aneurysm rupture has been estimated to be as high as 1–2% per year [4,13,28]. One of the most frequent cerebral aneurysms is an anterior communicating artery (ACoA) aneurysm, accounting for 23-40% of CA and 12-15% of unruptured aneurysms; this is the most common CA type in patients under 30 years [10,24]. Specific morphological factors, A1s, and A1 ratios have been shown to contribute to the hemodynamic behavior of the ACoA complex [14,27,30]. However, no study has examined the effect of an anterior cerebral artery (ACA) rotation degree on the presence of ACoAA.
Therefore, in the current study, we assessed the relationship between the degree of ACA rotation and the presence of ACoAA. We introduce the concept of the ACA rotation degree, which compares to the internal carotid artery line, and examine the possibility of the ACA rotation degree representing the importance of and acting as an independent predictive factor for ACoAA.
MATERIALS AND METHODS
Patients
After approval from our Institute Review Board (Blinded for review), a retrospective study of the database of ruptured or unruptured ACoA aneurysms (ACoAAs) before treatment either by microsurgical or endovascular techniques between 2015 and 2020 was carried out. Informed consent was waived due to retrospective design.
Patient data, such as age, sex, and social lifestyle, history of smoking, were collected, as were medical records such as hypertension. All patients underwent magnetic resonance angiography (MRA), and a three-dimensional (3D) program (Xelis, INFINITT, Korea) was used as a tool to measure the desired parameters, such as the ACA rotation degree, A1 axial diameter, A2 axial diameter, aneurysm size, and aneurysm angle. All patients were assessed for the A1 ratio, A2 ratio, and A1:A2 ratio after measurement. A study group and control Group B patient was included if he/she met the criteria, which were 18 to 75 years old and diagnosed with a single aneurysm, including an aneurysm size greater than or equal to 3 mm diameter. Exclusion criteria were multiple aneurysms, low image quality, arteriosclerosis, and cerebral vascular malformations.
Following the inclusion criteria, n=105 of the study group were collected. Hundred-five subjects at a ratio 1:1 to ACoAA, without any brain disease who visited our health center for medical check-ups, enrolled as control Group A, and who were diagnosed with a single middle cerebral artery (MCA) aneurysm before any treatment, enrolled as control Group B. Two distinct control group were selected as cases that match for sex and age at the same time of study group collection.
Definitions
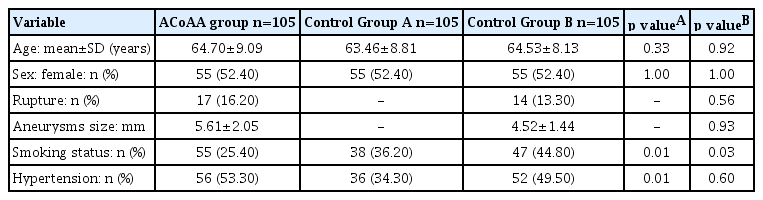
All parameters were measured with a particular definition, controlled by two professional neurosurgeons and neurosurgical trainees. ACA rotation was defined by the intersection of two particular lines ACoA and internal carotid artery (ICA). The posterior view of ACoAA in the 3D brain reconstruction MRA was rotated backward till the horizontal plan. The ACoA line was defined by the two most medial aspects of the beginning of both A2 segments, which is presented in Fig. 1. The ICA line is defined by the end of the most lateral part of the petrous part of the ICA, which is shown in Fig. 1.
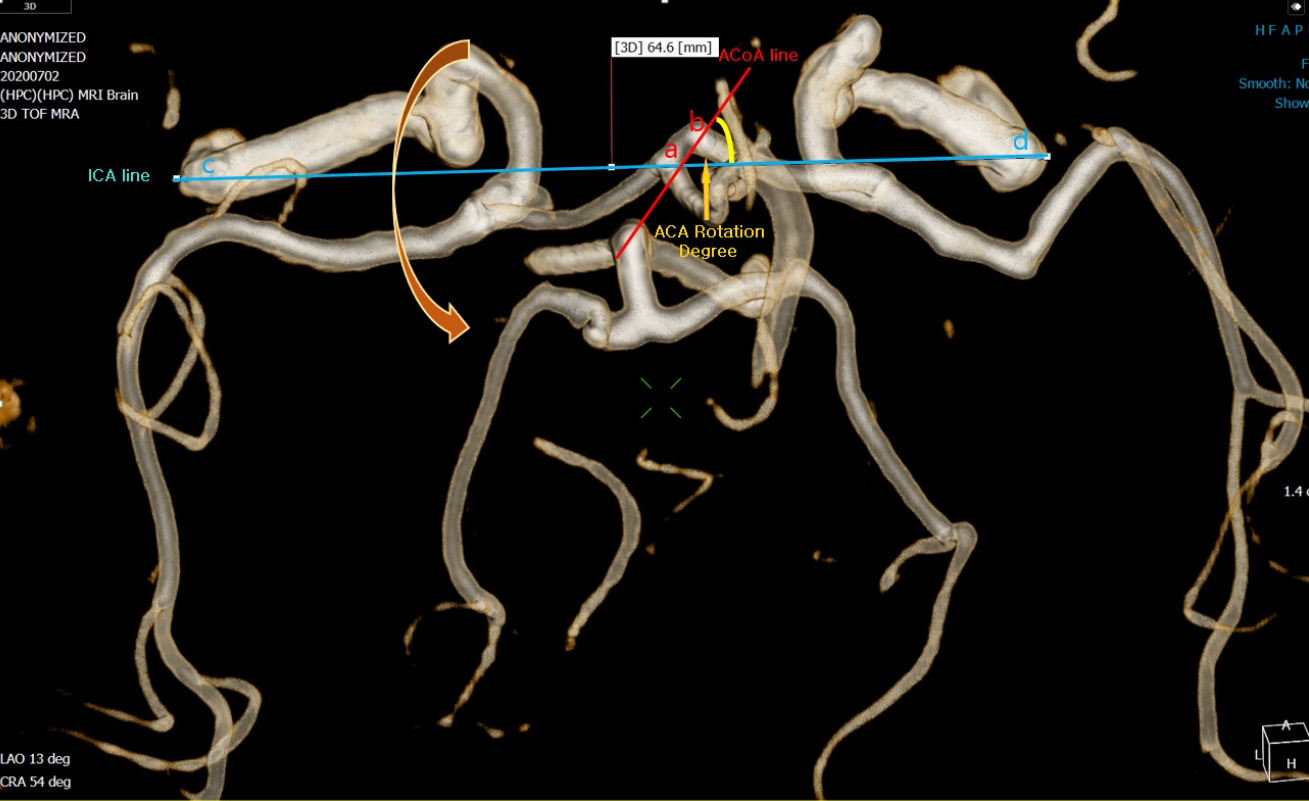
The definition of parameter uses in the ACA rotation degree. The red line represents the ACoA line, where “a” is the starting point of right A2 and “b” is the starting point of left A2. These lines are connected and create the ACoA line. The blue line represents the ICA line created by both lateral parts of the petrous part of the ICA. The ACoA line that crosses the ICA line at a particular point. These two lines create a particular angle “yellow line”. ACA, anterior cerebral artery; ACoA, anterior communicating artery; ICA, internal carotid artery
Another H-complex (composed of the ACoA, both A1s, and A2s) parameter, the A1 diameter, was measured at the vessel’s proximal segment at its widest point; the A2 diameter was obtained at the first measurable point within the vessel’s first 1 cm.5)15)21) The A1 segment was defined as symmetric when the cross-sectional diameter of A1 was less than 20% different. We classified asymmetry when crossing a sectional diameter of A1s was greater than 20% but less than 40% different. Hypoplasia was defined when the cross-sectional diameter of the A1s diameter was more than 40% different; aplasia was indicated when we could not measure it or it was undetectable or absent. The A1 or A2 ratio was calculated as the mean of A1 or A2 contralaterally subtracted ipsilaterally. The A1:A2 ratio was calculated by subtracting the mean A1 ratio from the mean A2 ratio.9) When A1s or A2s were aplastic, we calculated the A1 ratio or A2 ratio ipsilaterally.
Statistical analysis
Descriptive analysis was performed on continuous variables for all groups, including the mean, standard deviation, and range. The t-test or Mann–Whitney U, and multi-regression test were performed as indicated for categorical and continuous variables using available commercial software (IBM SPSS Statistics version 25, IBM Corp., USA). The Spearman coefficient test was used for the analysis of nonparametric data, with bivariate or partial independent continuous variables. Statistical significance was determined as a p value of <0.05.
RESULTS
A total of 315 subjects were included in the analysis. This study conducted a sex-age matching analysis of the ACoAA group and both control groups. We found that the rupture status of control Group B and the ACoAA group was statistically insignificant. The ACoAA group had an aneurysm size similar to that of control Group B. The ACoAA group had more frequent tobacco use than both control groups. Hypertension in the ACoAA group was insignificant compared to that in control Group B but significant compared to that in control Group A, as shown in Table 1.
ACoAA group vs. control Group A
The ACoAA group had a greater degree of ACA rotation than control Group A (46.18±30.37 vs. 34.44±22.30, p=0.002). The ACoAA group had a smaller A1 ratio and A1:A2 ratio than control Group A (0.34±0.38 vs. 0.72±0.29 and 0.40±0.50 vs. 1.12±0.24, with p values of p=0.0001 and p=0.0001, respectively), but the ACoAA group and control Group A had the same A2 ratio of 0.86. A1 asymmetricity in the ACoAA group was more frequent than that in control Group A (p=0.001). In addition, differences in A2 segments between the ACoAA group and control Group A were insignificantly different, as shown in Table 2.
ACoAA group vs. control Group B
Table 3 shows that the ACoAA group had a greater degree of ACA rotation than control Group B, but the difference was insignificant (46.37±30.29 vs. 40.48±22.98, p=0.110). The A1 ratio of control Group B was greater than that of the ACoAA group (0.75±0.25 vs. 0.34±0.38), and the A2 ratio of control Group B was greater than that of the ACoAA group (0.88±0.18 vs. 0.86±0.14), including the A1:A2 ratio (0.88±0.34 vs. 0.40±0.50), with p=0.0001, p=0.37 and p=0.0001, respectively.

Morphological characteristics of H-complexes in the ACoAA group and control Group B (middle cerebral artery aneurysm)
The ACoAA group had a significantly different A1 symmetricity (p=0.0001) than control Group B, with the former (16.2% vs. 63.8%) having less A1 asymmetry than the latter (18.1% vs. 22.9%). Nevertheless, A1 hypoplasia and A1 aplasia were more common in the ACoAA group than in control Group B, with percentages of 14.3% vs. 5.7% and 54% vs. 7.6%, respectively. The difference in the A2 segment category between the ACoAA group and control Group B was insignificant (p=0.33), and ACoAA patients had less A2 symmetry than control Group B patients (81% vs. 87.6%). Control Group B also had less A2 asymmetry than the ACoAA group (8.6% vs. 16.2%) but more A2 hypoplasia than the ACoAA group (3.8% vs. 1.9%).
ACoAA group subgroup analysis
In this study, we did not find any relationship between the ACA rotation degree of the ACoAA group and other aneurysm characteristics, such as aneurysm size (p=0.78) and aneurysm angle (p=0.83) as shown in Table 4. Correlations of the A ratio to the ACA rotation degree were insignificant (A1, p=0.56); all correlations were insignificant.
Predict the ACA rotation with A1, A2 morphology, and rupture status
Multiple regression was performed to predict ACA rotation from, aneurysm status, A1 morphology, and A2 morphology. These variables statically insignificantly predict ACA rotation, (p=0.9, R2=0.012). All variables added statically insignificantly to the precipitation (p=0.9).
DISCUSSION
H-Complex morphology has various anatomical variations that affect ACoAA formation. In this study, we found that the degree of ACA rotation was greater in the ACoAA group than in control Groups A and B. Indeed, A1 asymmetry was more frequent in the ACoAA group than in both control groups. There is strong morphological evidence that the ACoA complex shares a common feature in ACoAA formation, and we found higher A1 dominancy in the ACoAA group than in control Groups A and B. However, our current study shows insignificantly of ACA rotation degrees to A1 dominancy. In addition, researchers have found that the A1 ratio and A1:A2 ratio correlate positively with the presence of ACoAA when compared to data for two control groups (posterior circulation aneurysms) and a nonaneurysm group [11]. Other studies have focused on A1 asymmetry and the importance of a dominant A1 in ACoAA [7,18,19,27,29]. Evaluating the correlation between a certain angiographic pattern of flow in the anterior circulation and the clinical findings for 51 patients with ACoAA revealed the presence of A1 dominance, unilateral hypoplasia of a contralateral A1, and significant factors associated with aneurysm formation.
In ACoAA, hemodynamic factors play an important role in the development of aneurysms in the ACoA complex. Anatomical features are key factors in hemodynamic changes. Our present study found that the dominance of A1 morphology in the ACoA complex in the ACoAA group was higher than that in the control group. Recently, hemodynamic features of aneurysms and their vascular architectures have gained much attention [2,3,9,20]. The influence of blood flow on aneurysm development is an area of long-standing investigation and has been evaluated with newer technologies, including computational fluid dynamics and phase-contrast magnetic resonance imaging [1,6,17,25]. Another previous study reported asymmetric anterior cerebral artery anatomy, and ACoAA has been shown to develop via increased hemodynamic stress resulting from compensatory shunting of the blood through the communicating artery [14,26,27]. An early experiment by Hashimoto et al. found that ACoAA was induced by unilateral ligation of the common carotid artery. Flow-induced hemodynamic shear stress through an asymmetrical ACoA complex model is the course for endothelial and subsequent aneurysm formation.
As this is the first study of ACA rotation degree, we could not find any related studies for comparison, but we did find some similar studies in that the anterior communicating artery (ACoA) complex and orientation of the ACoA plane were investigated regarding surgical approach planning for ACoAAs [12,23]. In these studies, the length of the ACoA was in the transverse plane in half of the patients, following the classic description of ACoA anatomy; the average angle of right A1 dominant ACoAs and left A1 dominant ACoA was 42±6 degrees and 37±9 degrees, respectively. In conclusion, the main result of A1 dominance was the development of the A1-A2 junction on the dominant side, subsequent angulation of the ACoA plane in the transverse plane’s relation, and an angulation plane of the ACoA plane—in combination with the orientation of the aneurysm. Another study found that 63% of cases were A1-segment dominant and that the ACoA rotated toward the contralateral side [8]. Although the aim of this previous study was similar to ours, we sought to investigate the relationship between the ACoAA and the ACoA plane.
However, the present study did not find any relationship between the ACA rotation degree in the ACoAA group and other aneurysmal characteristics, such as aneurysm size, aneurysm angle, A1 ratio, and A2 ratio—including the A1:A2 ratio.
Limitations
This study had several limitations. First, this was a retrospective analysis of a limited number of patients at a single institute. Second, we measured the 2D angle of the 3D MRA generated by a construction program, such that the image might have interfered with the accuracy of the correct diameter estimation. Third, a parameter that was too small or could not be measured was considered aplasia in this study. We did not obtain genetic or pathologic data demonstrating the degree of ACA rotation, and subjects with a history of smoking and hypertension were included, which makes it quite difficult to differentiate the course of ACoAA from our objective research.
CONCLUSIONS
In our current study, the rotation degree of the ACA and A1 asymmetry were greater in the ACoAA group than in both control groups. The ACA rotation degree in the ACoAA group was significantly different from that in the nonaneurysm group. In a subgroup analysis of ACA rotation degree in ACoAA patients, we found a strong uncorrelation of the size of the aneurysm in ACoAA patients. Therefore, the rotation degree of the ACA is an independent risk factor for ACoAA and may serve as an independent imaging marker for ACoA aneurysms. However, there was no significant difference in the MCA group comparison, and further study is needed.
Notes
Disclosure
This work was supported by grant No. (032021014) from the SNUH Research Fund.