Efficacy of intraoperative neuromonitoring (IONM) and intraoperative indocyanine green videoangiography (ICG-VA) during unruptured anterior choroidal artery aneurysm clipping surgery
Article information
Abstract
Objective
The aim of this study was to investigate the efficacy of intraoperative indocyanine green videoangiography (ICG-VA) and intraoperative neuromonitoring (IONM) to prevent postoperative ischemic complications during microsurgical clipping of unruptured anterior choroidal artery (AChA) aneurysms.
Methods
We retrospectively reviewed the clinical and radiological records of all patients who had undergone microsurgical clipping for unruptured AChA aneurysms at our institution between April 2001 and December 2019. We compared the postoperative complication rate of the group for which intraoperative ICG-VA and IONM were utilized (group B; n=324) with that of the group for which intraoperative ICG-VA and IONM were not utilized (group A; n=72).
Results
There were no statistically significant differences in demographic data between the two groups. Statistically significant differences were observed in the rate of overall complications (p=0.014) and postoperative ischemic complications related to AChA territory (p=0.039). All the cases (n=4) in group B who had postoperative infarctions related to AChA territory showed false-negative results of intraoperative ICG-VA and IONM.
Conclusions
Preserving the patency of the AChA is essential to minimize postoperative complications. Intraoperative monitoring tools including ICG-VA and IONM can greatly contribute to lowering complication rates. However, their pitfalls and false-negative results should always be considered.
INTRODUCTION
The anterior choroidal artery (AChA) originates from the posterolateral wall of the internal carotid artery (ICA), and then passes through distal segments consisting of the cisternal and plexal segments, which are divided by the choroid fissure [27]. The AChA has perforating branches [19] and supplies eloquent areas including the posterior limb of the internal capsule, optical tract, lateral geniculate body, medial temporal lobe, and the medial area of the pallidum [40]. Aneurysms at the junction of the AChA and ICA account for 2–4% of all intracranial aneurysms. Treatment of AChA aneurysms can be challenging due to the particular conformation of the AChA, its small size, and the eloquence of the territory it supplies [1]. Infarctions related to AChA territory are characterized by the triad of contralateral hemiplegia, hemisensory loss, and homonymous hemianopsia [32]. Thus, preserving the patency of the AChA is important to avoid postoperative ischemic complications [20]. As devices and techniques advance, more AChA aneurysms are being treated using endovascular treatment modalities. However, patients with aneurysms that have broad necks or are large in size or incorporated in the AChA or endovascularly inaccessible are still being treated with microsurgical clipping.
Tools to preserve parent artery patency during microsurgical clipping have been developed, such as microvascular doppler sonography (MVDS), intraoperative indocyanine green videoangiography (ICG-VA), and intraoperative neuromonitoring (IONM) including somatosensory evoked potentials (SEPs) and motor evoked potentials (MEPs) [6,22,28,38,39]. At our institution, intraoperative MVDS was used during microsurgical aneurysm clipping before 2010, while intraoperative ICG-VA and IONM, along with MVDS, have been utilized during microsurgical clipping since then.
The aim of this study was to retrospectively investigate the efficacy of intraoperative ICG-VA and IONM to prevent postoperative ischemic complications during microsurgical clipping of unruptured AChA aneurysms.
MATERIALS AND METHODS
This retrospective study was approved by the institutional review board of Asan Medical Center.
Study population
We retrospectively reviewed the clinical and radiological records of all patients who had undergone microsurgical clipping for unruptured AChA aneurysms at our institution between April 2001 and December 2019. In total, 396 patients underwent microsurgical clipping during this period. We excluded (1) patients with subarachnoid hemorrhage and (2) those who did not undergo direct aneurysmal neck clipping such as coagulation or wrapping.
Clinical and radiological data collection and evaluation
Patient medical records on characteristics, aneurysm size, any postoperative neurological deficits, and postoperative radiological abnormalities were obtained through our institutional Electronic Medical Records (EMR) database. Patient characteristics included age, sex, hypertension (HTN), diabetes mellitus (DM), hyperlipidemia, and smoking status. Aneurysm size was defined as maximal dome size on three-dimensionally reconstructed images of preoperative computed tomographic angiography (CTA) or transfemoral cerebral angiography (TFCA). All patients underwent CTA immediately after surgery. In the absence of a focal neurologic deficit, patients routinely underwent brain CT on day 3 after surgery. Diffusion-weighted magnetic resonance imaging (DW-MRI) was evaluated to confirm postoperative infarctions in patients with any postoperative neurologic deficits. In asymptomatic patients, postoperative infarction was defined as a low-density area with a specific vascular supply territory on CT acquired on day 3 after surgery. Patients with symptomatic or asymptomatic infarctions underwent TFCA immediately to evaluate the patency of AChA and the parent artery. At the discretion of the surgeon, patients underwent postoperative TFCA to check the residual sac of the aneurysm.
Surgical procedures and outcome assessments
Conventional frontotemporal craniotomies following a pterional approach were conducted in all cases and clipping procedures were performed by the three experienced attending surgeons. The YASARGIL aneurysm clip system (Aesculap AG & Co., Tuttlingen, Germany) was used in all cases. Anterior clinoidectomy or exposure of the cervical ICA was performed if the aneurysm was large or low-lying or if proximal control was expected to be difficult due to severe atherosclerosis of the parent artery. Temporary clipping did not exceed 5 minutes in most cases. In all cases, MVDS was used to confirm the patency of distal flow in the parent artery and perforators after permanent clipping. IONM and ICG-VA were utilized for the procedure since 2010.
We compared the postoperative complication rate of the group for which intraoperative ICG-VA and IONM were utilized (group B) with that of the group for which intraoperative ICG-VA and IONM were not utilized (group A). Postoperative complications were divided into two categories, clinical and radiological. Clinical outcomes were evaluated using modified Rankin Scale (mRS) values at discharge and at 6 months after the surgery.
Anesthesia regimen
Total intravenous anesthesia was used in all cases, in combination with standard American Society of Anesthesiologists monitors. Following the injection of lidocaine (20–40 mg), anesthesia was induced with propofol (1.5–2.5 mg/kg). A commercially available 2-channel target-controlled infusion (TCI) pump (Orchestra, Fresenius Vial, Brezins, France) was used for effectsite TCI of propofol and remifentanil. Before the dural opening, anesthesia was maintained using continuous infusion of propofol (2.5 ug/mL) and remifentanil (7–9 ng/mL). After the dural opening, TCI rates were increased to 3.0 ug/mL for propofol and 10–15 ng/mL for remifentanil. Bispectral index monitoring (Aspect Medical Systems Inc., Framingham, MA, USA) was used to ensure adequate depth of anesthesia (target index range, 30–50). As elicited EP monitoring responses are very sensitive to suppression by muscle relaxants, full or partial neuromuscular blockage was not induced during microsurgical clipping with IONM. At the discretion of the anesthesiologist, a bolus dose of rocuronium (0.8–1.0 mg/kg) was administered only once to provide neuromuscular blockade for endotracheal intubation, or low-dose rocuronium was continuously infused under the guidance of a train-of-four (TOF) monitor [17]. The procedure was similar to that followed in our previous study [8].
EP monitoring
Preoperative SEP evaluations were conducted before the surgery, with a Neuropack MEB-2200 (Nihon Kohden, Tokyo, Japan). The baseline SEP and any abnormal amplitude or latency were used as the baseline reference during intraoperative monitoring. Preoperative MEP evaluations were not conducted, due to the pain caused by electrical stimulation during the evaluation. The same device was used intraoperatively by trained neurophysiologists. Subcutaneous, bilateral placement of cranial electrodes at C3/C4 was performed for both MEP and SEP monitoring according to the International 10-20 electroencephalogram system.
MEPs were elicited using a train of six constant-current anodal 0.5-msec-wide stimuli delivered at 3-msec interstimulus intervals. Bilateral MEPs were recorded using subcutaneous needle electrodes at the deltoid, abductor pollicis brevis, tibialis anterior, and adductor hallucis muscles. MEPs were recorded within a 150-msec interval with filtration and amplification (bandpass 30–2000 Hz, 10,000 times). The baseline stimulus intensity was tested at least three times, before the scalp incision, the craniotomy, and the dura incision. Once the baseline intensity was confirmed, the baseline amplitude of MEP/SEP waves was selected and recorded in the neurophysiology event log. SEPs elicited from the bilateral median and posterior tibial nerves were monitored throughout the surgery. The cranial electrode responses at C3/C4/Cz following continuous stimulation with a 4.7-Hz current at minimal stimulus intensity were used to assess SEPs.
The majority of previous studies on intraoperative EP monitoring suggest that a decrease in amplitude of greater than 50% should be considered a clinically significant change [12-15,17,21,33,36,37,41]. The neurophysiology team shared the EP results with the surgeon immediately in case of significant changes. MEPs were routinely recorded after the permanent clipping. Additional stimulations were conducted if there was a SEP decline or at the surgeon’s request. The final EP monitoring was recorded during the scalp closure, using MEPs. If significant EP changes occurred, the stimulation intensity was gradually increased and checked repeatedly. To avoid false-negative results, the stimulation intensity never exceeded 300 V. The procedure was similar to that followed in our previous study [8].
ICG-VA
After an uneventful surgical exposure and initial aneurysm clip placement, the surgeon requested ICG injection for VA monitoring. Patients were injected with 25 mg of ICG (DID Indocyanine Green; Dongindang, Inc., Gyeonggi-do, Korea) diluted in 20 mL of sterile water through the central venous catheter. VA monitoring confirmed the patency of distal flow in the parent artery and perforators and the presence of aneurysm sac filling.
Statistical analysis
The differences in demographic and clinical covariates between the two groups were examined with Student’s t-tests. Comparisons of outcomes were performed using the Fisher exact test or chi-square tests. All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp., USA). In this study, p values <0.05 were considered statistically significant.
RESULTS
From April 2001 and December 2019, 396 patients underwent microsurgical clipping of unruptured AChA aneurysms at our institution. Seventy-two patients (group A) underwent microsurgical clipping without IONM and ICG-VA, while 324 patients (group B) underwent microsurgical clipping with IONM and ICG-VA. Intraoperative MVDS was used in both groups. The demographic data of all patients are presented in Table 1. The mean age of the patients was 55.2 years in group A and 56.9 years in group B. The proportion of female patients was higher than that of male patients in both groups, but there were no statistically significant differences in sex or age between the two groups. Histories of hypertension, diabetes mellitus, hyperlipidemia, and previous smoke were also compared, but no statistically significant differences between the groups were found. The mean aneurysm diameter was 3.57 mm in group A and 3.33 mm in group B, with no statistically significant difference between the groups (p=0.26).

Baseline characteristics for the 396 patients who underwent microsurgical clipping of unruptured anterior choroidal artery aneurysms
Clinical and radiological outcomes are summarized in Table 2. Morbidity at 6 months (mRS≥3) was 1.4% and 0.6%, respectively (p=0.453). No mortality was observed in either groups. The overall postoperative complication rate after microsurgical clipping was 9.7% (seven patients) in group A and 2.8% (nine patients) in group B (p=0.014).
Group A contained five cases (6.9%) of postoperative ischemic complications. Among these, infarctions related to AChA territory were observed in four patients (5.6%). One patient had infarctions unrelated to AChA territory (1.4%), and this individual exhibited multifocal middle cerebral artery (MCA) territory infarctions due to postoperative vasospasm. Another patient had a postoperative intracerebral hemorrhage (ICH) in the right frontal lobe but did not show any postoperative neurologic deficits. The other patients exhibited transient third nerve palsy, which completely recovered within 6 months.
Group B contained nine cases (2.7%) of postoperative ischemic complications. Among these, infarctions related to AChA territory occurred in four patients (1.2%). However, no significant EP changes were found throughout surgery in any case and the patency of AChA flow was also confirmed by ICG-VA. Fig. 1 shows preoperative and postoperative TFCA, DW-MRI, and intraoperative ICG-VA images as well as the intraoperative MEP record of one patient with postoperative AChA territory infarctions.
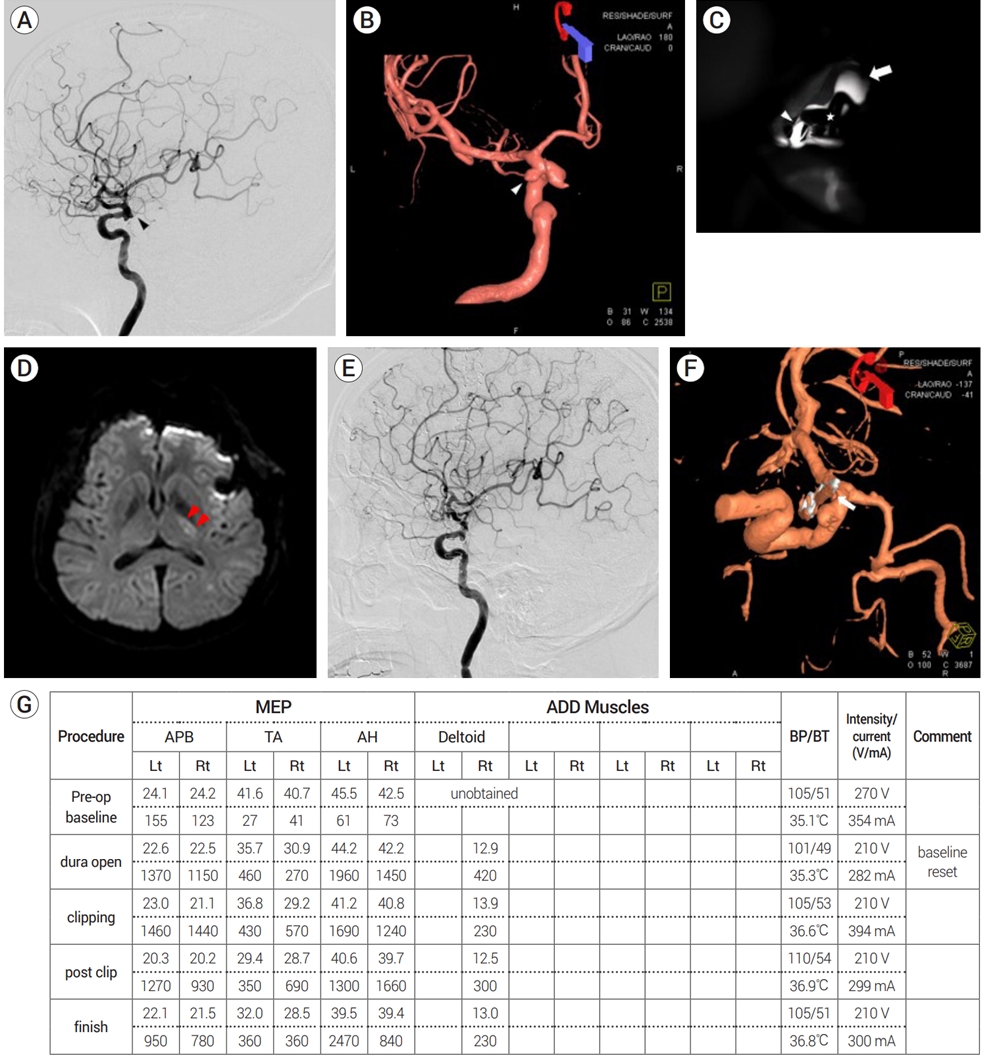
Images of a patient who underwent microsurgical clipping for an unruptured left anterior choroidal artery (AChA) aneurysm using a straight clip. (A) Preoperative transfemoral cerebral angiography (TFCA) showing the left AChA (black arrowhead). (B) Preoperative TFCA 3-dimensional image showing an unruptured left AChA aneurysm (white arrowhead). (C) Intraoperative indocyanine green videoangiography (ICG-VA) showing patent flow of left AChA after clipping. The left internal carotid artery (white arrow), the AChA (white arrowhead), and the clip (asterisk) are shown. (D) Postoperative diffusion-weighted magnetic resonance imaging (DW-MRI) showing an acute infarction in the left posterior limb of the internal capsule (red arrowheads). (E) The left AChA is not visible on postoperative TFCA. (F) Postoperative TFCA 3-dimensional image showing complete obliteration of the aneurysm. Note the atherosclerotic change of the parent artery and the small filling defect (white arrow) near the clip blade. This may represent vessel wall kinging or a dislodged atheroma causing obstruction of the distal flow. (G) No significant MEP changes were observed during the surgery. MEP, motor evoked potential
Infarctions unrelated to AChA territory occurred in five patients (1.5%). These patients had multiple aneurysms and showed postoperative infarctions related to aneurysms other than AChA.
Statistically significant differences were observed between the two groups in the rate of overall complications (p=0.014) and postoperative ischemic complications related to AChA territory (p=0.039). However, the rate of infarctions unrelated to AChA territory did not differ statistically between the groups (p=0.7). Complications other than infarctions (hemorrhage or CN III palsy) showed no statistically significant difference.
DISCUSSION
Anatomy of the AChA
The AChA usually arises from the supraclinoid portion of the ICA, distal to the origin of the posterior communicating artery (PComA), as a single artery. It may infrequently arise from the ICA as two separate arteries or as a single artery that immediately splits up into two trunks [26,30]. It rarely arises from the MCA or the PComA. The diameter of its origin is smaller than that of the PComA and similar to that of the ophthalmic artery. The AChA is divided into cisternal and plexal segments, with the cisternal segment ranging from the origin to the choroidal fissure and the plexal segment including branches that pass through the choroidal fissure and enter into the choroid plexus of the temporal horn. The artery arises postero-medially behind the ICA, and passes below or along the medial side of the optic tract toward the lateral geniculate body. At the anterior margin of the lateral geniculate body, it crosses the optic tract from medial to lateral and passes postero-laterally through the crural cistern. Then, it passes through the choroidal fissure and along the medial border of the choroid plexus. The branches from the cisternal segment commonly supply the optic tract, the lateral geniculate body, the posterior two-thirds of the posterior limb of the internal capsule, most of the globus pallidus, the origin of the optic radiations, and the middle third of the cerebral peduncle. The branches from the plexal segments commonly anastomose with branches of the lateral posterior choroidal arteries and supply the choroid plexus. There is substantial interchangeability of vascular territories among the AChA and the branches from the posterior cerebral artery (PCA), PComA and MCA [26,27,30].
Postoperative infarctions after AChA aneurysm clipping and surgical considerations
In previous studies, the incidence of postoperative infarctions after microsurgical clipping of AChA aneurysms was reported to be between 4.8% and 7% [4,16,18]. At our institution, before the use of IONM and ICG-VA, the incidence of postoperative infarctions (6.9%) was similar to that reported in the previous studies. However, after we started using INOM and ICG-VA, the incidence of postoperative infarctions decreased to a significant extent (2.7%).
Not only is the use of various intraoperative monitoring tools important, but it is essential to identify the origin and the course of AChA aneurysms during microsurgical clipping to prevent postoperative complications. At our institution, a mirror or endoscope was used to identify the origin of the aneurysm if it was hidden behind the aneurysm dome. If this method does not work, distal AChA patency can be verified occasionally in the supracarotid triangle, which is the space above the ICA bifurcation [31], as this space is relatively superficial and has no structures covering the AChA. However, the AChA is sometimes too small to be identified with MVDS. In such cases, ICG-VA is essential to confirm AChA patency after aneurysm clipping.
Intraoperative monitoring tools: advantages and disadvantages
Several intraoperative monitoring tools have been utilized during aneurysm clipping. At our institution, intraoperative MVDS was used solely until 2009. Although MVDS is non-invasive and time- and cost-efficient, it is occasionally unreliable owing to small vessel size or a suboptimal insonation angle [2,9]. To compensate for the shortcomings of MVDS, we introduced intraoperative ICG-VA and IONM after 2010. ICG-VA is minimally invasive, repeatable, easily performed, and has good resolution. Additionally, it shows vessel stenosis, not only as morphologic stasis but on a dynamic basis by presenting the flow delay in sequenced time frames [2]. However it has several disadvantages: (1) structures can only be enhanced and visualized under a microscope; (2) the detection and interpretation of the flow delay depend on the surgeon; (3) according to earlier reports, it prevents ischemia due to branch occlusion in only 94% of cases; and (4) the blood flow confirmed on ICG-VA can be compromised after the release of brain retraction [2,10,11,25].
The usefulness of SEPs and MEPs in aneurysm surgery is well known [2]. MEPs are more effective in detecting vascular insufficiency in the anterior circulation and deep structures than SEPs [2,22], and SEPs are thus generally used as the supplementary tool for MEPs. However, MEPs also have several limitations. First, they may result in patient movement, which can interfere with microsurgeries. Furthermore, the surgeon should consider the false-negative IONM results during the surgery. In the current study, we identified four false-negative cases with postoperative infarctions related to AChA territory after aneurysm clipping.
False-negative IONM and ICG-VA results
Previous studies have shown false-negative IONM results during microsurgical clipping [2,5,15,22,24,36]. Szelenyi et al. reported that the rate of false-negativity during clipping was about 7% [36], and Irie et al. reported a false-negative rate of about 5.4% [15]. Compared to these previous studies, our sample included only unruptured AChA aneurysms and had a lower rate of false-negativity (1.2%). Fig. 1 shows images of a patient with false-negative IONM and ICG-VA results in our sample. There are several possible explanations for these results. First, high-intensity stimuli of up to 1500 V may bypass a cortical or subcortical lesion and stimulate the motor tract distal to the lesion [22,29]. However, the stimulation intensity was kept below 300 V in our study. Second, a lesion that is not located in the corticospinal tract can result in postoperative motor deficits without intraoperative IONM changes. Suzuki et al. reported that infarctions in the premotor area may lead to motor deficits without significant EP changes [34]. However, the false-negative cases in our study showed postoperative infarctions in AChA vascular territory, as confirmed on DW-MRI. Other possible mechanisms include delayed perfusion due to hypotension, cerebral edema, and lacunar infarctions after the monitoring is terminated [7].
Four false-negative cases of postoperative infarctions related to AChA territory exhibited patent AChA flow on intraoperative ICG-VA. The reliability of ICG-VA could be adversely affected by brain shifting after the release of brain retraction, due to changes in the positional relationship between the clip and the adjacent vessels. This can cause trauma to the adjacent vessels, resulting in thrombosis and vessel occlusion [9]. Moreover, atherosclerosis near the aneurysm may result in postoperative ischemic complications after clipping [3,23,35]. Atherosclerotic changes in the parent artery may cause the clip to rotate and thus result in kinking of the adjacent vessels or slipping; even when distal flow patency is confirmed by intraoperative ICG-VA, this can lead to blood flow compromise when the brain retraction is released [24]. Additionally, atheromatous plaque ruptures or emboli from the atherosclerotic parent artery can cause lacunar infarctions during repeated clipping of the parent artery [23,24].
Limitations of this study
Since our study has a single-center retrospective design, there might have been a selection bias. Second, there was a discrepancy in the number of patients as well as the study period between the two groups. Third, as endovascular treatment has developed rapidly over the course of the study period, the indications of aneurysm clipping might have differed between the two groups. Fourth, the development of microscopes and MVDS might have affected the surgical outcomes of the two groups. Therefore, multicenter prospective studies are needed to avoid selection biases in the evaluation of the effectiveness of intraoperative monitoring tools during microsurgical clipping of AChA aneurysms.
CONCLUSIONS
Postoperative infarctions related to AChA territory after aneurysm clipping result in severe neurologic deficits including hemiplegia, hemisensory loss, and homonymous hemianopsia. Thus, preserving the patency of the AChA is essential to minimize postoperative complications. Intraoperative monitoring tools including MVDS, ICG-VA, and IONM contribute greatly to lowering the complication rate. However, their pitfalls and false-negative rates should always be considered.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.

