De-novo formation of neighboring intracranial aneurysm after spontaneous thrombosis of a ruptured distal anterior cerebral artery aneurysm: A case report and review of the literature
Article information
Abstract
Intracranial aneurysms arise in 1-2% of the population and usually present as hemorrhagic strokes. Spontaneous thrombosis of a ruptured intracranial aneurysm occurs in 1-3% and most commonly in giant aneurysms, with complete thrombosis in just 13-20% of the cases. Thrombosis of smaller aneurysms is rare. Here we present a case of a patient who presented with a ruptured intracranial aneurysm that subsequently thrombosed, discovering a neighboring de-novo aneurysm during follow-up. We hypothesized that after thrombosis, the hemodynamic characteristics that contributed to the formation of the first aneurysm were replicated.
INTRODUCTION
Intracranial aneurysms arise in 1-2% of the population and represent 85% of non-traumatic subarachnoid hemorrhages cases and 10% of hemorrhagic strokes [2]. Spontaneous thrombosis of a ruptured intracranial aneurysm occurs in 1-3%, is generally reported in giant (>2.5 cm) aneurysms [4, l5,16] and usually partially or incompletely, presenting complete thrombosis in just 13-20% of the cases [3,4]. Thrombosis of smaller aneurysms after rupture is unusual, as it is without rupture [14]. These completely thrombosed aneurysms can recanalize spontaneously, can present with ischemic stroke via embolism or occlusion of the parent artery or can decrease in size (resorption) [6–8,11]. Some of the mechanisms associated with the thrombosis of ruptured intracranial aneurysms are the presence of an aneurysm volume to neck area ratio >28.3 [1], with those aneurysms with a narrow neck being more prone to thrombosis due to slow flow and stagnation, increasing viscosity and platelet aggregation. Roach in 1978 proposed that aneurysms with a dome-to-neck ratio >4 were susceptible to thrombosis, while those with a ratio <2.5 were prone to rupture [12]. Other factors associated with thrombosis are female sex, size >10 mm, localization in the anterior circulation, stasis of contrast material during angiography, and endothelial injury, possibly due to increased wall shear stress [11,14].
Here we present the case of a male patient who presented with a ruptured intracranial aneurysm that subsequently thrombosed, discovering a newly formed neighboring aneurysm. A comprehensive review of the available published literature was conducted on PubMed of all papers that contained the keywords “intracranial aneurysm,” “de novo formation,” “risk factors,” “thrombosis,” published up to October 2021. All images and digital imaging and communication in medicine (DICOM) analysis and reconstruction were performed with Horos, a free and open-source code software (FOSS) program distributed free of charge under the LGPL license at Horosproject.org and sponsored by Nimble Co LLC d/ b/a Purview in Annapolis, MD USA.
CASE DESCRIPTION
We present the case of a 51-year-old male with no family history of aneurysms, a smoking habit since he was 17 years old, and no chronic diseases. He presented to the emergency department with the “worst headache in his life,” nausea, vomiting, and diplopia without changes in his mental state. A angiography computed tomography (AngioCT) scan was performed, and a diagnosis of aneurysmatic subarachnoid hemorrhage mFisher II, Hunt, and Hess II was made, secondary to the rupture of a saccular distal anterior cerebral artery aneurysm (DACA); at that moment, no neurological deficit was noted. He was admitted to the intermediate care unit and started on antihypertensive drugs and nimodipine. He was offered surgical treatment, but due to personal motives, he refused, so after a couple of uneventful weeks of medical surveillance, he was discharged. Two months later, he came for a neurosurgical consultation in the outpatient clinic and accepted surgical treatment. A new AngioCT was performed to visualize the morphology of the saccular aneurysm, noting the presence of an area of encephalomalacia in the right medial frontal lobe and a decrease in the size of the aneurysm, from 5.7 mm to 0.96 mm in neckdome length with a 2.07 mm neck, in the exact apparent location, so an angiography was done, corroborating the presence of the DACA aneurysm but confirming its decrease in size. Due to the high suspicion of thrombosis, a magnetic resonance imaging (MRI) study was requested, visualizing an area of encephalomalacia and a circular hypointense lesion in the distal anterior cerebral artery at an early bifurcation of the pericallosal and calloso-marginal branches were noted (Fig. 1).
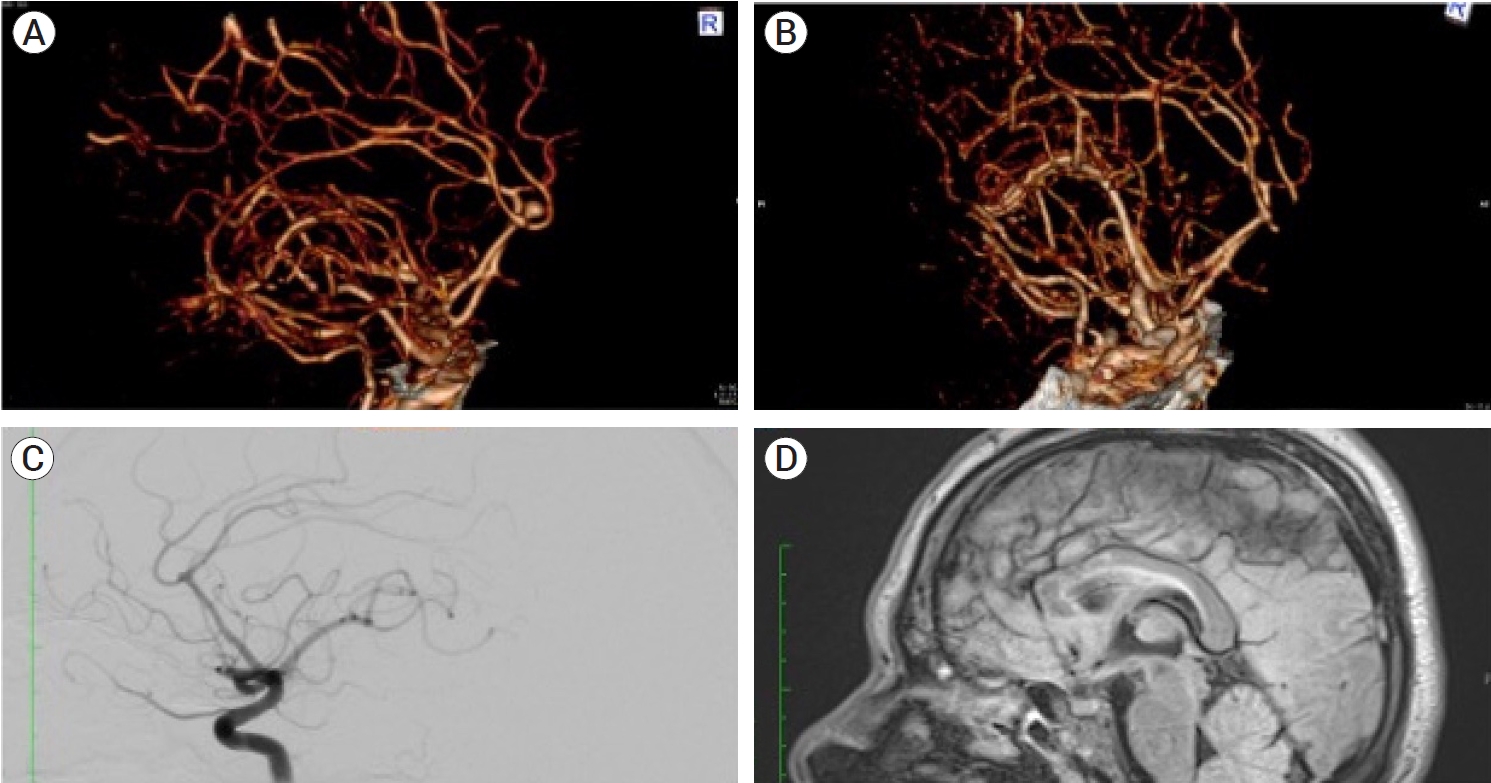
Preoperative images of the patient demonstrate the presence of a saccular aneurysm in the right distal anterior cerebral artery. (A) 3D AngioCT reconstruction at the time of rupture performed in the emergency department demonstrates the presence of the DACA aneurysm in the vertical portion of the interhemispheric cistern. (B) 3D reconstruction of the AngioCT performed two months later, noting the decrease in the neck-to-dome length. (C) A lateral projection of the angiography corroborating the presence of the aneurysm at the right DACA bifurcation, with its decrease in size from the original AngioCT to the one performed two months later. (D) Pre-operative MRI, a T2 sequence in a sagittal plane, in which a saccular lesion dependent of the right DACA is visualized, bigger than the one visualized in the AngioCT and Angiography. DACA, distal anterior cerebral artery aneurysm; AngioCT, angiography computed tomography; MRI, magnetic resonance imaging
After discussing the risks of rebleeding versus vigilance, he was scheduled for intervention, with a diagnosis of a thrombosed DACA aneurysm with clipping as the primary objective. A mini interhemispheric approach was performed, as previously described by Monroy-Sosa et al. [9], after interhemispheric dissection of the vertical portion of the interhemispheric cistern, both pericallosal arteries were visualized. The right pericallosal artery was followed proximally to its known early bifurcation where the aneurysm was located according to the pre-op image studies, finding a big thrombosed aneurysm and a side-to-side (or neighboring) baby aneurysm (Figs. 2 and 3). Both aneurysms were excluded from the circulation with the application of Yasargil clips. After the surgery, the patient was discharged on postoperative day 3 with no new neurological deficit.
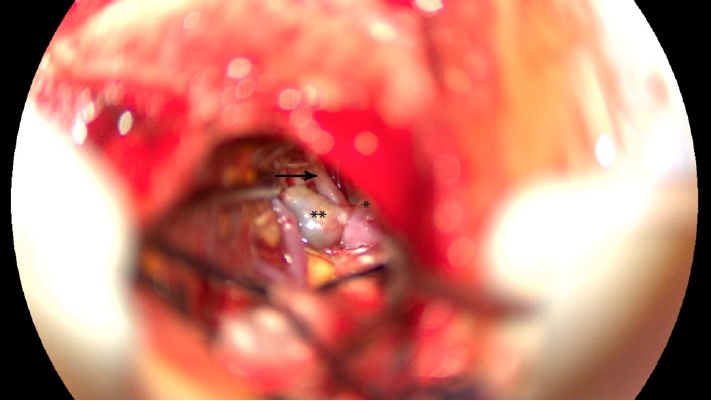
Intraoperative image using the surgical microscope in which the presence of the thrombosed aneurysm (**) and the neighboring de novo aneurysm (*) are noted. The previously ruptured aneurysm is thrombosed, and the pericallosal artery (solid arrow) and callosomarginal artery branching from the anterior cerebral artery are visualized. After this, both aneurysms were clipped.
DISCUSSION
Saccular aneurysms occur in 1-2% of the population and represent 85% of non-traumatic subarachnoid hemorrhages; 85% of the aneurysms are located in the anterior circulation, and approximately 20% have more than one aneurysm. These lesions are more common in women with a 3:1 ratio, are associated with polycystic kidney disease, hypertension, and a family history of ruptured intracranial aneurysms [2]. Cerebral aneurysms usually present with subarachnoid hemorrhage [4]. However, thrombosis, either total or partial, is a common outcome in giant aneurysms, but not as usual in small ones [11,16], noted in some series to present in up to 76% of those bigger than 2.5 cm [7]. Some of the geometric factors and hemodynamic mechanisms proposed in the presentation of these aneurysms with thrombosis instead of stability, growth or rupture are the volume/orifice ratio (volume-neck ratio), volume to area quotient, contrast stasis during angiography, aneurysm (or inclination) angle, vessel angle, and aneurysm-to-vessel ratio [1,4,7,10–12,16].
The Dome-to-neck aspect ratio has been associated as a crucial geometric risk factor for thrombosis. It produces a low shear rate, diminished pulsatile flow, promoting microenvironment changes at the aneurysm wall with proinflammatory and procoagulant cascades being amplified [14]. Roach suggested that those aneurysms with a ratio >4 were more likely to rupture [12].
In our case, the aneurysmatic dome-to-neck ratio was 2.7 (below 4), and the volume to neck surface was 26.44 (below the originally described 28 threshold, but above the usual 25), with partial risk for thrombosis.
During the procedure, the previously ruptured aneurysm was found completely thrombosed, and a baby aneurysm, not noted in the original AngioCT scan, was present at its side. We called this a neighboring aneurysm. Both aneurysms were clipped, and no residual lesion was found in post-op studies.
The pooled incidence rate for de-novo aneurysm formation is 0.34% and 0.09% for unruptured and ruptured aneurysms, respectively, with a median time of diagnosis to de novo formation of 8 years for a single aneurysm [13]. Our patient presented with common risk factors for the formation of the de novo lesion, such as smoking and age [13,15].
We propose that the mechanism for the formation of the new baby aneurysm is related to the complete thrombosis of the previously ruptured one at a neighboring location, simulating the same geometric factors and hemodynamic environment for the formation and rupture of the first one. Unfortunately, at the moment of the angiographic procedure, the hemodynamic analysis software was not available, so our hypothesis could not be proven.
CONCLUSIONS
We report a case of a de novo baby aneurysm secondary to the rupture and complete thrombosis of a neighboring distal anterior cerebral artery aneurysm. To our knowledge, there is no other report of a case like the one we present.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.

