An interesting case of survival to multiple ruptures of aneurysms, with persistent trigeminal artery, cranial nerve deficit, and evolutionary exposure of neurovascular treatment
Article information
Abstract
Subarachnoid hemorrhage secondary to rupture of an aneurysm is a severe condition, associated with a high rate of morbidity and mortality. There are few cases in the literature of rupture of an aneurysm of the persistent trigeminal artery. This is the case of a 62-year-old female who has suffered multiple ruptures of aneurysms, in different decades of her life, with the development of De novo aneurysm, been this the presented case, a rupture of aneurysm of the persistent trigeminal artery. This patient has survival to these conditions and remain without important morbidity. The case manifested with a clinical picture of third and seventh cranial nerve deficit, which this last one, there are not previous publications of cases with this deficit. This aneurysm was embolized with coils, and the postoperative condition was satisfactory, been discharged at 4 postoperative days.
INTRODUCTION
An intracranial aneurysm is a dilation on the wall of a cerebral artery, which in most cases occurs in the circle of Willis [18]. Spontaneous subarachnoid hemorrhage is a serious condition that develops after the rupture of an intracranial aneurysm. The prevalence of this pathology ranges between 1% and 5%. On some occasions, patients with intracranial aneurysms may develop a second aneurysm, different from the primary location. This second aneurysm is called a “De novo aneurysm” [5]. Due to the early age of onset and high mortality, subarachnoid hemorrhage accounts for more than 25% of years potential life lost for all stroke victims under the age of 65 [18].
CASE DESCRIPTION
History
A 62-year-old female who suddenly developed a 10/10 holocranial headache, associated with nausea and multiple episodes of vomiting, for which she was taken by her relatives to the emergency department of the Complejo Hospitalario Dr. Arnulfo Arias Madrid (CHDrAAM) in Panama City. The patient had not manifested any history of fever, head trauma or activities related to Valsalva maneuvers. She had not previous neurological deficit, had not suffered from similar conditions, neither had she presented changes in her body weight. In 1974 she debuted with a similar picture, and was taken to medical attention and within her studies the presence of a cerebral aneurysm was evidenced, requiring surgery. Later, in 1998, she had another clinical picture with the same clinical characteristics, and another cerebral aneurysm was found during her evaluation, and an aneurysm clipping was performed. She had no personal pathological medical history, nor consumption of substances of abuse.
Examination
Upon evaluation, she was hemodynamically stable, in good general condition, without alterations in her general physical examination. On neurological evaluation, she was alert, conscious, and oriented. Nuchal rigidity was confirmed. New onset complete right third cranial nerve (CN) palsy and right House-Brackman (HB) 3 peripheral facial palsy were observed. The rest of the cranial nerves had no alterations. Sensitivity in all its modalities was preserved. Muscle strength was 5/5 in all four limbs, tendon reflexes were ++ globally, and muscle tone was normal. Likewise, gait and cerebellar functions were normal. She had no pathological reflexes.
Pathological findings
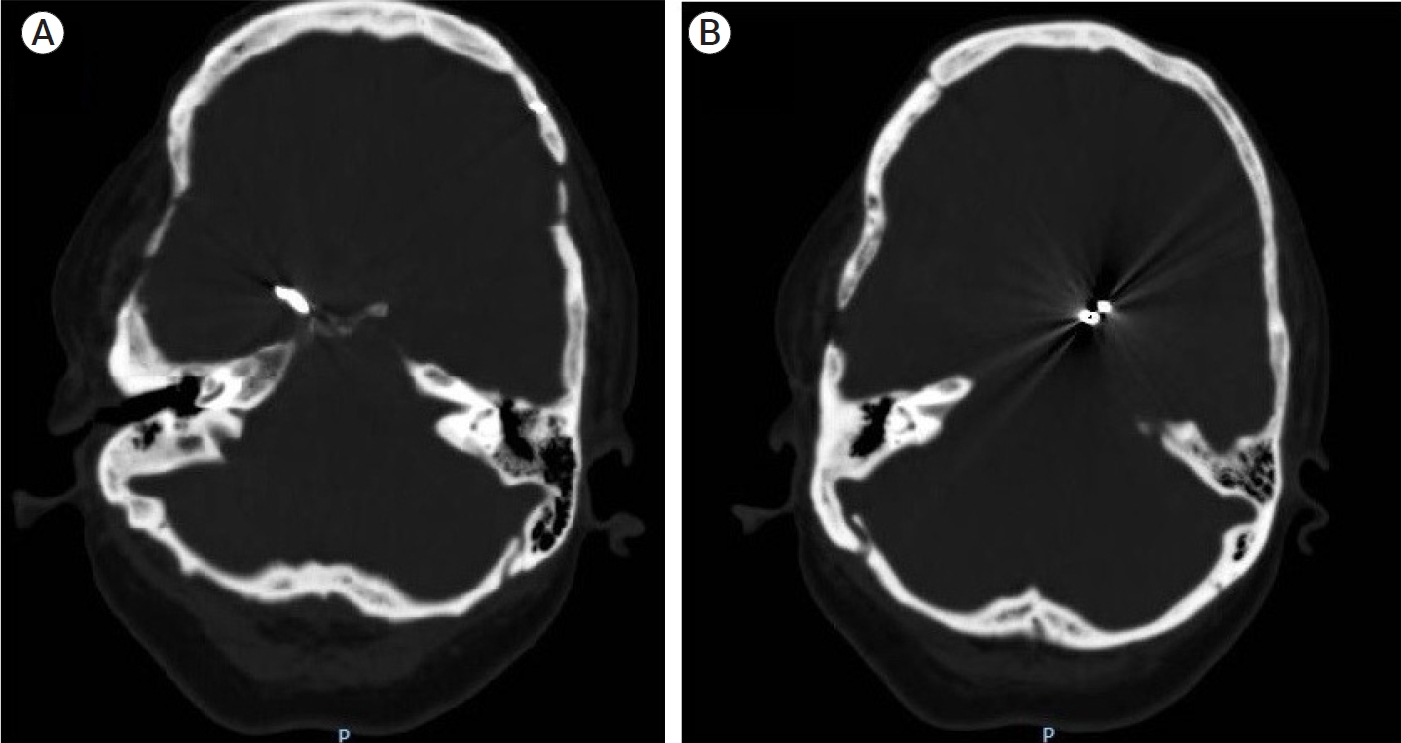
A head computed tomography (CT) scan was performed, which revealed signs of subarachnoid hemorrhage (SAH) at the level of the suprasellar cistern, without drainage to the ventricular system or associated hematoma (see Fig. 1). In addition, the presence of a simple vascular clip (malleable) was observed in what seemed to be the territory of the left internal carotid artery, and a modern clip (spring-loaded) in the territory of the right posterior communicating artery (see Fig. 2).

Head computed tomography (CT) scan. (A) Subarachnoid hemorrhage is observed at the level of the suprasellar cistern. (B) There is no evidence of drainage of blood to the ventricular system.
Treatment
She was admitted as a Fisher 3 – Hunt & Hess 2 subarachnoid hemorrhage. She was transferred to the intensive care unit for hemodynamic and neurological monitoring, as well to start vasospasm prophylaxis.
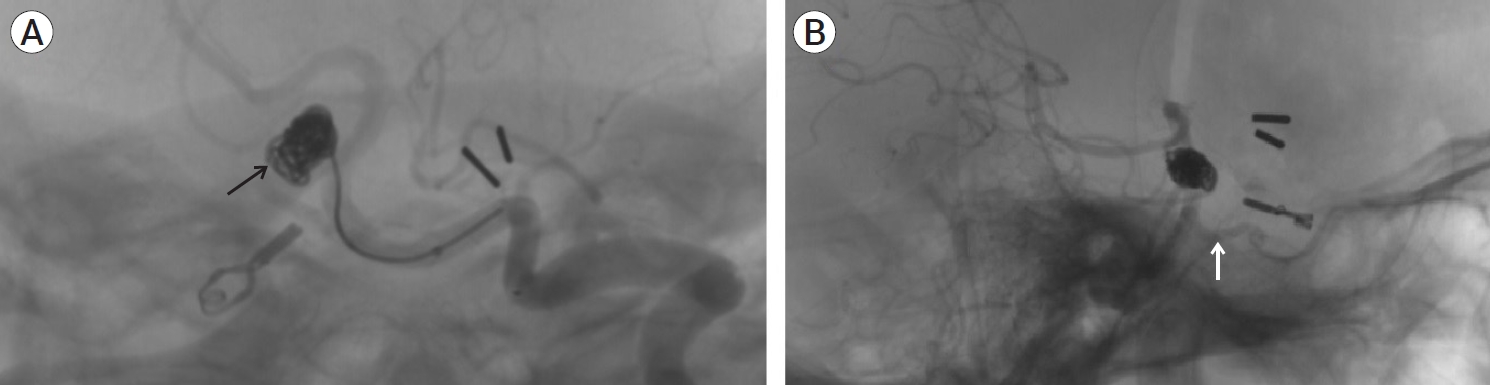
Cerebral angiography was performed, wish showed a completely clipped aneurysm of the right posterior communicating artery. A trigeminal artery was observed, originating from the upper third of the basilar artery and communicating with the petrous segment of the right internal carotid artery, providing collateral circulation. A saccular aneurysm was identified at the origin of the trigeminal artery with dimensions of 7.2×8.5 mm and a neck of 4 mm (see Fig. 3). Embolization was performed with the use of two 5 mm×13 cm Penumbra® coils (CA, USA), achieving a satisfactory occlusion and keeping the trigeminal artery permeable (see Fig. 4).

Cerebral angiography (3D reconstruction). Image (A) shows the panoramic view, with a cross indicating the aneurysm with dimensions of 7.2×8.5 mm and a neck of 4 mm. (B) Anteroposterior view (note the relationship of the aneurysm with the anatomical exit of the third cranial nerve and a caudal twist of the persistent trigeminal artery, which could explain the III and VII cranial nerve palsy, respectively). (C) Side view. Note the anterior orientation of the patent trigeminal artery toward the petrous portion of the internal carotid artery. Cross and yellow line: aneurysm. White arrow: persistent trigeminal artery.
Postoperative course
The patient evolved hemodynamically stable in the intensive care unit, without deterioration in her neurological status and was discharged after 4 postoperative days. One year later, the patient is in good general condition, with 80% recovery from the third cranial nerve deficit, and from peripheral facial paralysis to HB-2.
DISCUSSION
The development of intracranial aneurysms is currently controversial. Risk factors that predispose to the development of this pathology have been described. Among these risk factors there is the genetic or hereditary pattern, where pathophysiological mechanisms related to vascular endothelial maintenance have been described and, on the other hand, hereditary syndromes or high-risk conditions [11,16].
In the case presented, the patient did not have a hereditary-family history that could link her to a probable risk factor, neither she suffered from predisposing syndromes or substance use.
Approximately 500,000 people die of aneurysm rupture worldwide [11,16]. Despite a steady reduction in SAH mortality in recent years, this entity is still associated with considerable morbidity and mortality. In acute cases, 10 to 25% of all patients die immediately after bleeding [12], in subacute states it can increase to 50% due to rebleeding and chronically to 75% mortality [9]. This means that 25% can remain, either dependent on nursing care or living independently [16]. In cases of developing vasospasm, these figures increase drastically [2]. These data indicate the high mortality and morbidity that this pathology presents, and allows us to emphasize the survival of the case presented, having three ruptured aneurysms at different ages.
A study conducted by Fingerlin TJ et al. retrospectively analyzed 21 patients who had at least 10 years of follow-up after brain aneurysm surgery. In this study, they found a De novo aneurysm formation rate of 23.8% over an average period of 13.1 years (annual incidence of 1.8%). Other studies have reported development rates between 0.37% and 4.15% for each year. It has been described that the risk of De novo aneurysm rupture is 2.9% per year, and that young, female patients with multiple aneurysms are at high risk of developing these aneurysms De novo. [4] Risk factors and time for De novo aneurysm formation are not well studied, so there is no follow-up protocol to determine them. In the present case, the patient had two of these risk factors, being female and presenting her first aneurysm rupture at a young age, however, when her first aneurysm was detected, she only had one, and not multiple. Despite the absence of an established protocol, the follow-up of young women with or without multiple aneurysms could be favorable, and pose it as a research question.
Intracranial aneurysms are characterized by a pathological structure of the wall with rupture of the internal and medial elastic lamina, which leads to focal pockets of weakened arterial wall [13]. The most common type of aneurysm is the saccular, which is associated with a thin or absent tunica media and an absent or highly fragmented internal elastic lamina. Approximately 85% of aneurysms are located in the anterior circulation, predominantly in the junctions or bifurcations along the circle of Willis [6]. In the case presented, a saccular aneurysm was identified in relation at the origin of a persistent trigeminal artery, being this the third aneurysm found in this patient. It is also noteworthy is the location of the aneurysm, which was found in the posterior circulation, despite the fact that they are more frequently found in the anterior circulation.
The persistent primitive trigeminal artery is a fetal carotid-basilar anastomosis. It is the most common embryological vascular remnant that persists into adulthood [15]. The literature suggests that there is an association between cerebral aneurysms and vascular variations, being among these variations the carotid-basilar anastomoses, and an association of up to 29% being reported. Some believe that it is an incidental association and others that it is more than causal [19].
The formation of aneurysms located in the trigeminal artery is associated with structural and/or hemodynamic factors. However, it can be attributed to selection bias, to the high incidence of their simultaneous existence in the same patients. At present, it is still unclear whether these associations are incidental or inevitable [7].
The trigeminal artery has been classified by Later Salas et al. in 2 types: lateral (petrous) and medial (sphenoidal) and by Saltzman in 3 types. The first type, according to Saltzman, is the classic trigeminal artery that directly connects the internal carotid artery with the basilar artery. The second type is the trigeminal-cerebellar arteries, a variant that purely supplies the cerebellum. This type can also be classified into three subtypes based on their vascular territories (the superior cerebellar artery, the anterior inferior cerebellar artery, and the posterior inferior cerebellar artery). The third type is the trigeminal artery incorporated with the other two embryonic arteries, i.e., the primitive stapedial artery and the primitive ophthalmic artery, resulting in various complex arterial variants (opthalmo-steped-trigeminal, stapedo-trigeminal and staped-trigemino-cerebellar anastomosis) [7]. In our case, the patient presented the classic type according to Saltzman and the lateral or petrous type according to Later Salas.
Some of the signs that the patient manifested in the present case were third right cranial nerve (completely), and seventh cranial nerve palsy (peripheral HB-3). It has been described that approximately 8% of cases of intracranial aneurysms can manifest with cranial nerve palsy [16], and this occurs mainly in large or giant aneurysms that produce a mass effect [1]. Only the presence of a persistent trigeminal artery, can cause complete third cranial nerve palsy [7], of the sixth cranial nerve [14], even with trigeminal neuralgia [17]. In our review, we did not find other reports of cases of persistent trigeminal artery aneurysms in which seventh cranial nerve palsy was observed.
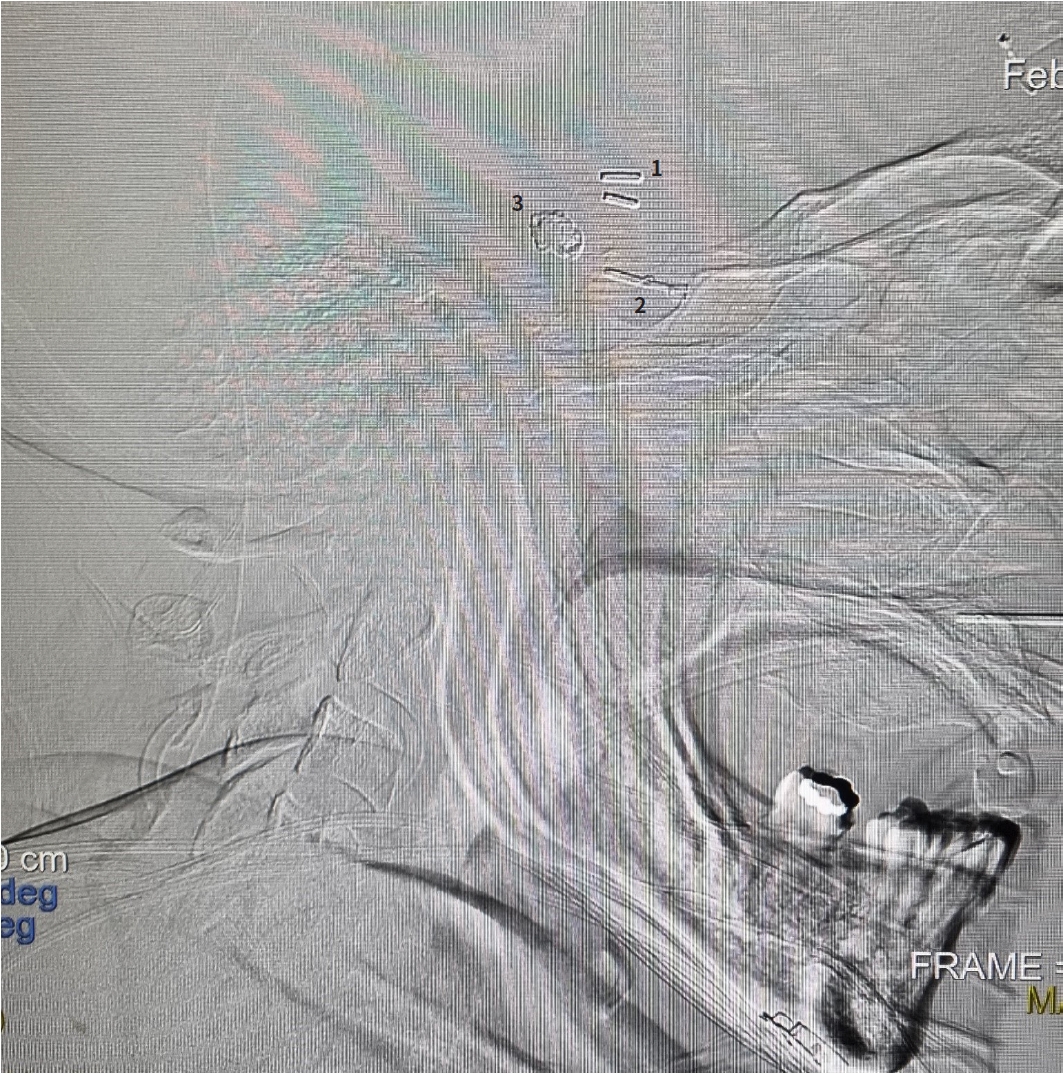
The management of intracranial aneurysms has been constantly evolving. The concept of a proximal vascular ligation to induce thrombosis in an aneurysm was introduced in 1748. However, the first case of aneurysm treatment using a clip (Silver clip) was performed in 1911 by Harvey Cushing. Then, in 1950, the era of modern aneurysm clips began with the introduction of the Mayfield-Kees clip, and in 1977 the Yasargil clips were developed, which are the most widely used today [8]. Later, in 1990, the endovascular management using a platinum coil was introduced, however, it was not until 2002 when it began to gain worldwide acceptance [10]. Surprisingly, this patient presents the three milestones for treating intracranial aneurysms: with a simple vascular clip (malleable) in 1974 (before the introduction of the Yasargil clip), in 1998, already established the Yasargil clip, and recently, in 2021 with endovascular management through embolization with coils (see Fig. 5).
CONCLUSIONS
This case shows a patient with a variant, a rare vascular abnormality, with multiple aneurysms at different stages, with a peculiar clinical manifestation, who surprisingly survived three different aneurysm ruptures, avoiding, thanks to neurosurgical development, the deadly advance of the pathology, and allowing a historical exposition to the different treatments of intracranial aneurysms. Adding to this, according to the available literature, this case represents the tenth report of a ruptured aneurysm of the persistent trigeminal artery with subarachnoid hemorrhage [3,17,19].
Acknowledgements
To the Dr. Anastasio Ameijeiras for the review and advise in the preparation of this case.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.