 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 24(4); 2022 > Article |
|
Abstract
A 35-year-old female presented with episodes of frequent dizziness, ear fullness, and right ear tinnitus for 12 months. Head imaging revealed a right glomus tympanicum tumor. She underwent pre-operative endovascular embolization of the glomus tympanicum tumor with surgical, cyanoacrylate-based glue. Immediately after the procedure, she developed drowsiness and severe pain in the right temporal region. Further investigations revealed a right cerebellar stroke in the posterior inferior cerebellar artery territory. She was treated with intravenous heparin, followed by one year of oral anticoagulation. With rehabilitation, she significantly recovered from her post embolization stroke. However, the tumor was resected at another institution. Ten years later, follow-up imaging indicated a gradual increase in the size of the glomus jugulare tumor compressing the nearby critical vascular structures. She subsequently received radiation therapy to treat the residual tumor. Currently, she has no neurological deficit, but her mild dizziness, right ear tinnitus, and hearing impairment persist.
Although rare, glomus tumor (i.e., nonchromaffin chemodectomas and paragangliomas) is the most common middle ear tumor, with female predominance [6]. It is a benign, slow-growing lesion thought to arise from the JacobsonŌĆÖs nerve at the cochlear promontory. The most common clinical presentation includes tinnitus, otalgia, and conductive hearing loss. The long-standing nature of symptoms often prompts further investigations with non-invasive imaging. On computed tomography (CT) scan, they appear as soft tissue mass located lateral or anterior to the cochlear promontory, the ossicles might be encased or displaced, and bone erosion might be seen. In the case of the large size of the tumor, they may fill the middle ear cavity and invade the Eustachian tube or mastoid air cells. Most of these tumors are hypervascular and histologically demonstrates numerous vascular channels in a background of a fibrous matrix with an impacted nest of cells separated by a membrane [10]. Their typical appearance on the magnetic resonance imaging (MRI) is of ŌĆ£salt-andpepper,ŌĆØ which reflects the multiple flow void of the larger vascular channels in the tumor [8].
Management of such tumors often involves a multi-disciplinary team approach. Often, a combination of treatments is used for the best outcomes. One such accepted treatment option is endovascular embolization of the tumor, followed by surgical resection. Pre-operative embolization is often required to devascularize the hypervascular tumor for better surgical outcomes. Embolization of such tumors is often tedious and requires careful planning, considering the location of the tumor and possible dangerous anastomosis between the feeding arteries with other innocent arteries supplying vital structures in the brain. Another challenge of embolization is the inadvertent spill of embolization material into the non-target vessels, leading to dire consequences [1]. One of such anastomosis can affect the posterior circulation and cause the reflux of the embolization material particles, such as in our patient.
A 35-year-old female without any significant past medical history was diagnosed with a right glomus tympanicum tumor during workup for the feeling of aural fullness and right ear tinnitus associated with frequent episodes of dizziness for 12 months. The ear tinnitus was pulsatile in nature and not whooshing, buzzing or hissing. With time her hearing on the right ear decreased, and she developed a mild unsteady gait. She had no nausea, vomiting, or other neurological symptoms. Her symptoms virtually disappeared in the subsequent eight months, though this was followed by a recurrence, requiring admission. Notably, she had no past surgical history. Her mother and sister have hearing difficulties. She was divorced, a mother of one child, housewife. She was awake, alert, and oriented, and her vital signs were normal. The neurological examination at the time of diagnosis revealed decreased hearing on the right side and minimal ataxic gait. Otoscopy examination revealed a red, smooth, pulsatile lesion behind the tympanic membrane. Her baseline audiogram showed a bilateral sensorineural hearing loss, mild to severe on the right ear, and mild on the left at some frequencies, otherwise normal.
The case report was approved by an institutional review board that waived consent due to the anonymous scanned files review.
The initial laboratory results were within normal ranges. CT scan of the temporal bone with contrast showed a small enhancing soft tissue lesion at the right hypotympanum consistent with glomus tympanicum and no evidence of glomus jugulare mass. Focal dehiscence of the left superior semicircular canal was noted. An MRI with magnetic resonance angiography (MRA) and magnetic resonance venography (MRV) of the brain and an MRI of the temporal bone and internal auditory canal with contrast again demonstrated a soft tissue signal intensity in the middle ear cavity of the right side with intense enhancement (Fig. 1). The ipsilateral jugular vein and the internal carotid artery were unremarkable. Digital subtraction angiography indicated a hypervascular lesion with tumor blush within the expected area of the right middle ear cavity supplied by one of the right ascending pharyngeal artery branches, likely the inferior tympanic artery (Fig. 2). As a part of the multi-disciplinary management plan, she underwent pre-operative embolization of the tumor. During the embolization procedure, super-selective catheterization of the right ascending pharyngeal artery was performed using a microcatheter, and an angiogram was performed to assess any dangerous anastomosis before embolization. Subsequently, (Fig. 3) embolization was performed using a 20% concentration of glubran injected through the microcatheter in the inferior tympanic artery (Fig. 4). Immediately after the procedure, the patient experienced severe drowsiness and vomiting. Urgent brain CT was performed, which indicated reflux of the glue material into the right vertebral artery with small scattered hyperdensities at the region of the posterior cerebral arteries and the right posterior inferior cerebellar artery (PICA) distally (Fig. 5). The neurologic examination did not reveal any new cranial nerves deficit or weakness, though the patient was still drowsy and complaining of dizziness.
We gave her heparin infusion as per ischemic stroke protocol. The following day, the patient developed a mild headache. A follow-up brain CT showed occlusion of the vertebral artery just proximal to the basilar junction and acute infarction along the inferomedial aspect of the right cerebellar hemisphere in PICA territory with hyperdense lesions related to the reflux of embolization material. A brain MRI confirmed acute right cerebellar stroke in the PICA distribution (Fig. 6) and complete occlusion of the right vertebral artery just proximal to the basilar junction (Fig. 7). She improved clinically with significantly reduced headache during her hospital course, and dizziness decreased with independent ambulation. Heparin infusion was switched to oral warfarin, and she was discharged home in stable condition. On follow-up she had mild persistent headache and dizziness. She subsequently underwent partial surgical resection of the right glomus tympanicum tumor at an outside institution. Continued follow-up with imaging showed a progressive increase in the size of the residual tumor.
Her intermittent headaches on the vertex and dizziness to a milder degree reportedly persisted on further follow-ups. Warfarin was discontinued after one year. She was lost to follow-up and returned after seven years. She had a tumor resection in a private hospital during that period, but no complete surgery details were available. The bilateral hearing impairment, tinnitus, and otalgia had persisted. A follow-up otology examination revealed regrowing reddish mass behind the right tympanic membrane. A follow-up brain CT and an MRI/A with MRV of the brain and the internal auditory canal revealed right glomus jugulare that was obstructing the flow along the right internal jugular vein and causing slow venous flow in the right transverse sinus but no evidence of venous thrombosis (Fig. 8). A tumor board meeting recommended external beam radiation therapy (EBRT). A brain MRI with neuronavigation and contrast was performed, and 12 Gray stereotactic radiosurgery (SRS) was administered to the right glomus. The patient developed vertigo and vomiting as side effects of the SRS. She remains stable with mild dizziness, right ear tinnitus, and bilateral hearing impairment without focal neurological deficit. The latest hearing assessment showed significant deterioration of hearing on the right ear with a profound sensorineural hearing loss (dead ear).
The name glomera jugulare was first used by Guild in 1941 when he was describing a structure at the jugular bulb that looks similar to the glomus body [4]. The first use of the term glomus tumors in English literature was in 1945 by Harry Rosenwasser [11]. More attention was given to this group of tumors after that; based on a review of 23 patients, Oldring and Fisch published their classification for glomus tumors and gave a guideline of the surgical approaches [9]. Fisch classification divided the glomus tumor to; type A, B, and C. Type A tumor that is limited to the tympanic region called glomus tympanicum tumors. Type B tumors involve the middle ear and extended to the mastoid process without destroying infralabyrinthine temporal bone. Type C tumors are those with infralabyrinthine extension and usually have an extension toward the petrous apex and can cause destruction of infralabyrinthine [9].
The term chemodectoma was used for glomus tumors as they assumed that they originate from the chemoreceptors of the blood vessels. However, they appeared to be derived from the neural crest, originate from nonchromaffin paraganglionic cells, and act as chemoreceptors [7].
The management of glomus tumors can be complex and requires a careful evaluation of the tumor and complex local anatomy, particularly the blood supply and feeders [12]. They are best managed with a multidisciplinary team, including head and neck surgery, neurosurgery, neurology, otology, oncology, and endovascular services. Early literature showed suboptimal treatment of these tumors with partial surgical debulking of the lesion, typically with radiotherapy [2]. The management of these complex tumors evolved with advances in imaging, microsurgical techniques, radiation therapy, and endovascular techniques. The Mayo Clinical protocol was one of the early protocols that were used to treat glomus tumors [3]. With the availability of multi modalities that include microsurgical dissection, radiation therapy, and embolization, these tumors become feasible to most patients. Considering the hypervascular nature of tumors, upfront surgery is often suboptimal due to extensive intra-operative bleeding. With advanced endovascular techniques and improved embolic agents, endovascular embolization is preferable before surgical resection of these tumors. Endovascular embolization can be performed in isolation or as an adjunct to other treatment modalities. Embolization aims to devascularize the tumor to minimize intra-operative bleeding, which helps achieve maximum safe resection. Embolization can be performed before radiation therapy to improve the efficacy of radiation therapy by cytoreduction and thus to aid in reducing the tumor volume to minimize the mass effect on the adjacent neuronal tissue [5]. Although embolization is a safe procedure, it is not without risks and can carry significant complications. Most complications occur during the procedure due to the inadvertent flow of embolic agents into non-target vessels. This can happen either through an artery to artery anastomosis or due to reflux of embolic agent into other branches. In the glomus tympanicum, the most common arterial feeders arise from ascending pharyngeal artery (APA). It is a branch of the external carotid artery with extensive arterial networks. Particularly important during the embolization procedure is the anastomosis of APA with anterior and posterior intracranial circulation. APA divides into two separate divisions, pharyngeal branch and posteriorly located neuro-meningeal trunk. The neuro-meningeal trunk further divides into the jugular and hypoglossal branches. The most common anastomosis between APA and posterior circulation happens through the hypoglossal artery, which can vary from a persistent hypoglossal artery directly filling the basilar artery to small anastomotic channels filling either vertebral artery or PICA. These small anastomotic channels are often not readily recognizable, even on super-selective angiograms. In our patient, small anastomotic channels were present between the hypoglossal artery and PICA which were not visible on the super-selective angiogram. During embolization through the APA branches, no reflux of embolic agent into these anastomoses was appreciable, which may be attributed to bony structures along the skull base hampering the visibility of embolic agents in this region. Most utilized embolic materials include particulate agents and liquid agents [e.g., N-butyl cyanoacrylate (glue) and ethylenevinyl alcohol copolymer (onyx)].
This case highlights the complex nature of these tumors which often bring challenges to the patients as well as treatment teams. Multi-disciplinary team approach is necessary to tailor management plan for individual tumors. Although embolization is a safe procedure, careful attention and thoughtful anatomic knowledge regarding dangerous anastomosis is essential to avoid devastating complications. Complications occur due to encountered vessel anomalies and new anastomoses formed during the gluing and changes in hemodynamics. Persisting symptoms after the surgical treatment warrant careful and vigilant follow-up to detect early tumor progression.
ACKNOWLEDGEMENTS
We are deeply indebted to King Fahad Medical CityŌĆÖs nursing staff for their excellent care provided to the patients. In addition, we appreciate all the specialties involved in this patientŌĆÖs multidisciplinary care, especially neurology, neurointervention radiology, neurosurgery, ENT, radiation oncology, audiology, and nevertheless, the ancillary services of Medical Imaging and Laboratory departments.
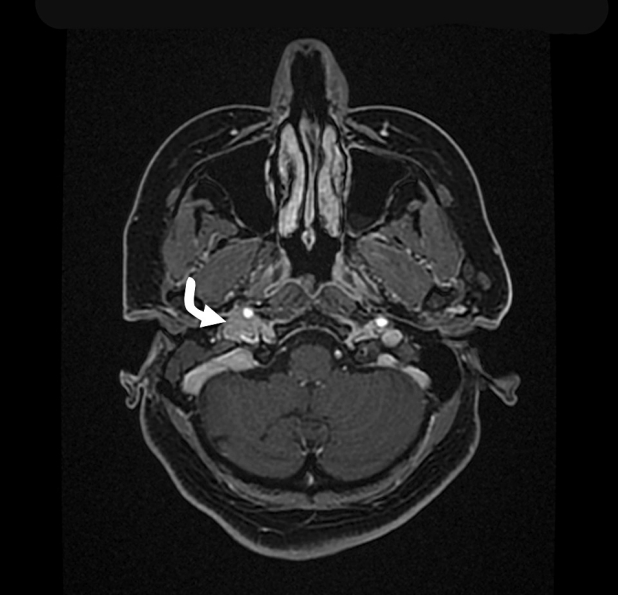
Fig.┬Ā1.
Magnetic resonance imaging (MRI) Axial 3D brain volume (BRAVO) of temporal bone and internal auditory canal with contrast demonstrates a soft tissues signal intensity (curved white arrow) in the middle ear cavity of the right side with intense enhancement.
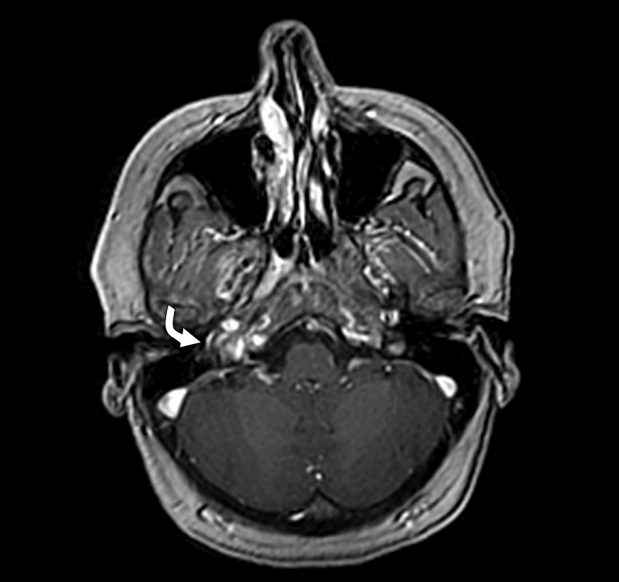
Fig.┬Ā2.
Cerebral angiogram shows tumor blush (white circle) is at the expected area of the right middle ear cavity that is supplied by one of the branches of right ascending pharyngeal artery likely inferior tympanic artery.

Fig.┬Ā3.
Cerebral angiogram, selective catheterization of the right ascending pharyngeal artery reaching nearly the tumor bed (white circle).
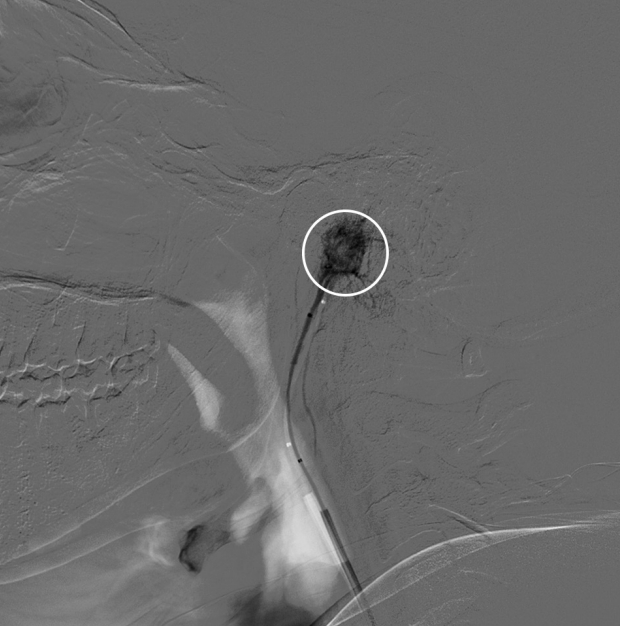
Fig.┬Ā4.
Cerebral angiogram post embolization with 20% concentration of glubran without residual tumor blush (white circle).
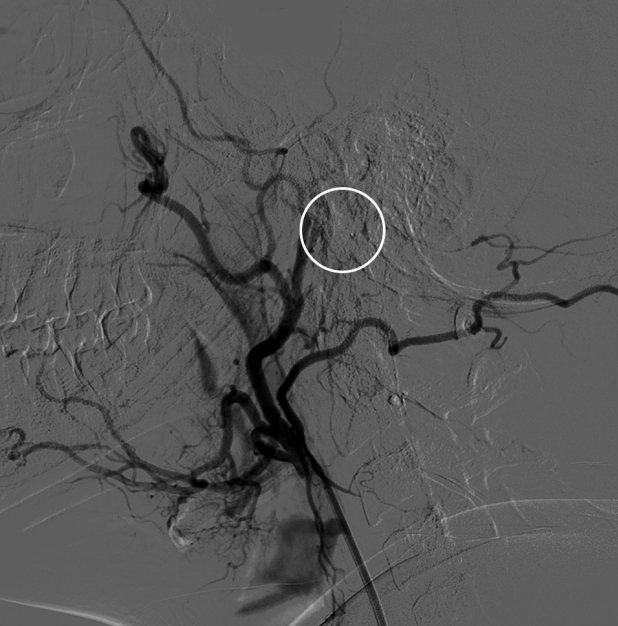
Fig.┬Ā5.
Computed tomography brain plain post embolization demonstrates glue material seen at the region of the right temporal bone (red arrow) with small reflux of glue material to the right vertebral artery (white arrowhead).
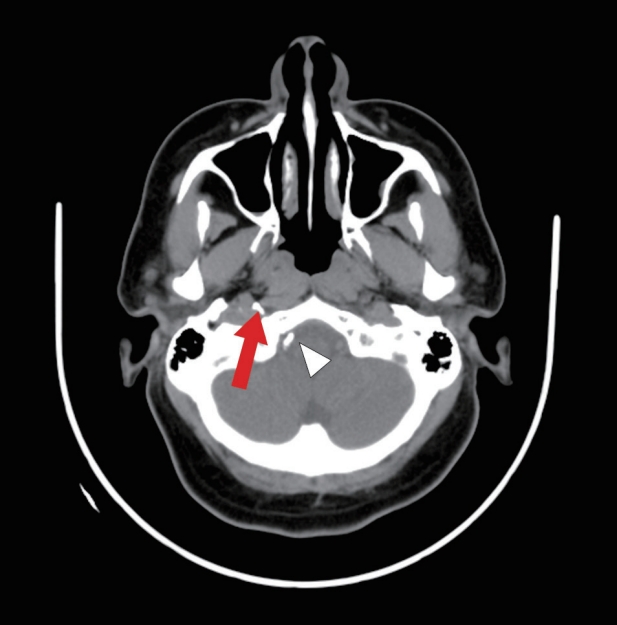
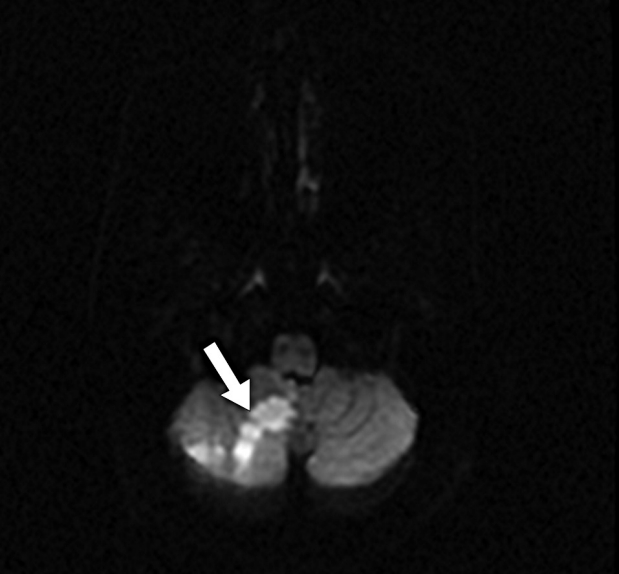
Fig.┬Ā6.
Brain MRI post embolization shows acute right cerebellar stroke in the PICA distribution (straight white arrow). MRI, magnetic resonance imaging; PICA, posterior inferior cerebellar artery
REFERENCES
1. Burdick TR, Hoffer EK, Kooy T, Ghodke B, Starnes BW, Valji K, et al. Which arteries are expendable? The practice and pitfalls of embolization throughout the body. Semin Intervent Radiol. 2008 Sep;25(3):191-203.



3. Erickson D, Kudva YC, Ebersold MJ, Thompson GB, Grant CS, van Heerden JA, et al. Benign Paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001 Nov;86(11):5210-6.


4. Guild SR. A hitherto unrecognized structure, the glomus jugularis, in man. Anat Rec. 1941 79(2 Suppl):28-107.
5. Gupta R, Thomas AJ, Horowitz M. Intracranial head and neck tumors: endovascular considerations, present and future. Neurosurgery. 2006 Nov;59(5 Suppl 3):S251-60; discussion S3-13.



6. Larson TC, Reese DF, Baker HL, McDonald TJ. Glomus tympanicum chemodectomas: radiographic and clinical characteristics. Radiology. 1987 Jun;163(3):801-6.


7. Mulligan RM, Chemodectoma in the dog. Am J Pathol. 1950 26:680-1.
8. Noujaim SE, Pattekar MA, Cacciarelli A, Sanders WP, Wang AM. Paraganglioma of the temporal bone: role of magnetic resonance imaging versus computed tomography. Top Magn Reson Imaging. 2000 Apr;11(2):108-22.


9. Oldring D, Fisch U. Glomus tumors of the temporal region: surgical therapy. Am J Otol. 1979 Jul;1(1):7-18.

10. Olsen WL, Dillon WP, Kelly WM, Norman D, Brant-Zawadzki M, Newton TH. MR imaging of paragangliomas. AJR Am J Roentgenol. 1987 Jan;148(1):201-4.


- TOOLS
-
METRICS

-
- 2 Crossref
- 0 Scopus
- 2,016 View
- 48 Download
- ORCID iDs
-
Naim I. Kajtazi

https://orcid.org/0000-0002-9983-3584 - Related articles
-
Giant cerebellar cavernous malformation in children: A case report and literature review




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



