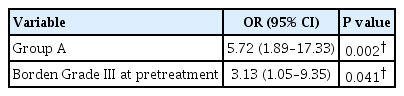Middle meningeal artery: An effective pathway for achieving complete obliteration following transarterial Ethylene Vinyl Copolymer (Onyx) embolization of dural arteriovenous fistulas
Article information
Abstract
Objective
Transarterial Onyx embolization is the mainstay of intracranial non-cavernous dural arteriovenous fistulas (dAVFs) treatment. Although the dural arterial supply varies depending on the location, the impact of arterial access on treatment outcomes has remained unclear. The aim of this study was to characterize factors as sociated with complete obliteration following transarterial Onyx embolization, with a special focus on arterial access routes and dAVF location.
Methods
A retrospective analysis of the patients who underwent transarterial Onyx embolization for intracranial dAVFs at two academic institutions was performed. Patients with angiographic follow-up were considered eligible to investigate the impact of the arterial access on achieving complete obliteration.
Results
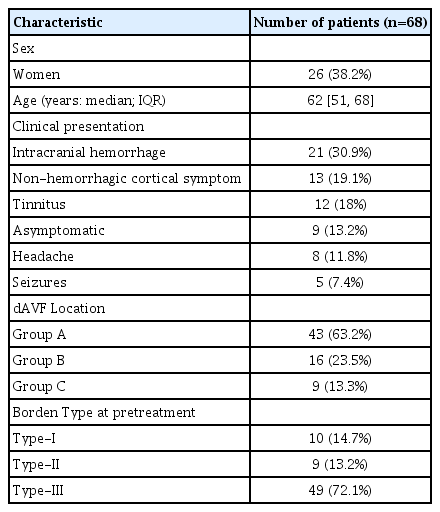
Sixty-eight patients underwent transarterial Onyx embolization of intracranial dAVFs. Complete obliteration was achieved in 65% of all treated patients and in 75% of those with cortical venous reflux. Multivariable analysis identified middle meningeal artery (MMA) access to be a significant independent predictive factor for complete obliteration (OR, 2.32; 95% CI, 1.06-5.06; p=0.034). Subgroup analysis showed that supratentorial and lateral cerebellar convexity dAVFs (OR, 5.72, 95% CI, 1.89-17.33, p=0.002), and Borden type III classification at pre-treatment (OR, 3.13, 95% CI, 1.05- 9.35, p=0.041), were independent predictive factors for complete obliteration following embolization through the MMA.
Conclusions
MMA access is an independent predictive factor for complete obliteration following transarterial Onyx embolization for intracranial non-cavernous dAVFs. It is particularly effective for supratentorial and lateral cerebellar convexity dAVFs and those that are Borden type III.
INTRODUCTION
Intracranial dural arteriovenous fistulas (dAVFs) can be satisfactorily treated with transarterial embolization through the arteries supplying the dura in the era of widespread availability of the ethylene-vinyl alcohol copolymer liquid embolic system (Onyx, Medtronic, ev3, Irvine, CA, USA) [2,3,4,7,9].
The middle meningeal artery (MMA) is the major supply of the cranial dura. It usually enters the cranium through the foramen spinosum and gives rise to anterior, petrous, petrosquamous and convexity branches in varying patterns. The dura located over the entire cerebral convexity, lateral segment of the cerebellar convexity, and falx cerebri, are supplied by convexity branches of the MMA. The arterial supply of the dura covering the medial cerebellar convexity is reinforced from the posterior meningeal artery. The dural supply to the cranial base is variable. The internal carotid artery supplies the medial aspect of the anterior and middle cranial fossa, as well as the anterior aspect of the posterior fossa. The external carotid artery together with branches of the MMA supplies the lateral aspect of all three cranial fossae [8].
The arterial supply to the dura is a key factor in determining the origin of feeding arteries in any given dAVF location. Selecting the appropriate arterial access route for embolization could potentially influence the likelihood of achieving a successful treatment outcome. The aim of this study was to characterize the factors associated with complete obliteration of dAVFs following transarterial Onyx embolization, with a special focus on arterial access routes and dAVF location.
METHODS
Study design, setting and participants
We performed a retrospective review of patients with intracranial dAVFs from 2 major academic institutions in the United States. The clinical data pertains to consecutive patients diagnosed with an intracranial dural arteriovenous fistula between January 1st 2009 and July 31st 2019. Patients with intracranial dAVFs treated with transarterial Onyx embolization as the primary modality were included in the study. Patients were considered ineligible if the lesion was initially treated with transvenous embolization or surgery. Patients treated via transarterial access with embolic agents other than Onyx were excluded. This study received institutional review board approval from each institution prior to its commencement. Informed consent was not obtained due to the retrospective nature of the study.
Variables, data source/measurements and quantitative variables
Medical records and angiographic imaging studies were reviewed for collection of patient demographics, clinical presentation, angiographic features of the dAVFs, location, Borden classification [1], arterial access route, treatment particularities, angiographic and clinical outcomes.
Complete fistula obliteration was strictly defined as complete disappearance of early venous drainage on follow-up digital subtraction angiography (DSA). Recurrence of dAVFs was diagnosed when there was detection of early venous drainage at the dAVF location, regardless of the degree of flow. Any given patient was considered cured when complete fistula obliteration was verified on a follow-up DSA usually performed between 3-6 months after treatment. Thereafter, cured patients were followed with magnetic resonance imaging/angiography (MRI/A) or computed tomography angiography (CTA). Angiographic results of the procedure were recorded with the Borden classification, by the treating interventionalists (C.O., A.P., and A.T.) and an independent neurosurgeon that was not involved in the procedure (Y.A.). Clinical evaluations were performed during follow-up clinical visits. Any procedure-related complications, including any unanticipated adverse event, if present, was recorded. Post-operative morbidity was defined as any permanent neurological symptom or change in the modified Rankin Scale (mRS) score ≥1 from pre-treatment to post-treatment values.
Transarterial Onyx embolization technique
All patients were treated under general anesthesia. After placement of a 6 French guiding catheter, we performed a coaxial or triaxial navigation of Onyx-compatible microcatheters into the feeding arteries of the dAVF with the intention of reaching as close as possible to the fistulous pouch. Onyx injection was performed using the subtraction road-map technique under fluoroscopic guidance. In cases of persistent Onyx reflux, the injection was paused for up to 2 minutes prior to reinitiating. The immediate angiographic outcome was evaluated with the DSA.
Data analysis and statistical methods
Quantitative variables are expressed as the median value and its interquartile range (IQR). The angiographic outcome was categorized as either complete or incomplete obliteration. In the univariate analysis, continuous variables were compared using a student’s t-test or a non-parametric assessment with the Kruskal-Wallis test, according to data distribution. Categorical variables were compared using the chi-square test. To evaluate for independent predictive factors for complete fistula obliteration, a multivariable logistic regression model was built, using a linear mixed effects model to account for within patient correlations, and using variables with a P value <0.10 on univariate analysis, accounting for any given collinearity and potential confounders.
Lesions were categorized into three groups depending on the dAVF location correlating to the distribution of the arterial supply to the dura mater [6,8]. Group A comprised lesions of the supratentorial and lateral cerebellar convexity dura (transverse sinus, torcular, falx, superior sagittal sinus and anterior fossa dAVF in the present series). Group B included lesions located in the dura of the anterior or middle cranial fossae, as well as the anterior portion of the posterior fossa (tentorial and petrosal dAVF). Group C consisted of lesions located in the paramedial or medial part of the dura covering the cerebellar convexity (posterior fossa dAVFs). Additionally, the arterial access was categorized as either MMA or non-MMA, to evaluate the impact of different arterial access on obtaining a complete fistula obliteration. A minimum of 20 observations were included for each variable in the model to account for overfitting. Statistical significance was set at P<0.05. All analyses were conducted using Stata 15.0 (StataCorp, College Station, Texas, USA).
RESULTS
Participant characteristics and descriptive data
A total of 97 dAVFs were identified in 95 patients. Of these, transarterial Onyx embolizations were performed as the primary modality of treatment in 68 dAVFs from 68 patients. Intracranial hemorrhage or focal neurologic deficits were observed in one half of the patients as the initial clinical presentation. The transverse sinus was the most common anatomical location (transverse sinus, 36.8%), followed by tentorium (25%), posterior fossa (13.2%), supratentorial convexity (8.8%), torcular sinus (7.4%), anterior cranial fossa (5.9%), falx and superior sagittal sinus (1.5% each). Accordingly, group A location was the most common (n=43), followed by group B and C (n=16 and n=9, respectively). Fifty-eight (85.3%) of these lesions had cortical venous reflux. Further details, including the proportion of lesions based on Borden type, are shown in Table 1.
Procedure-related outcomes
A total of 119 sessions of transarterial Onyx embolization were performed for 68 dAVFs (average of 1.75 sessions per dAVF). The prevalence rate for each feeding artery according to the dAVF location can be seen in Table 2. In group A, the MMA was the most frequent feeding artery (81.4%). While in group B, the MMA and the occipital artery (OA) were common feeding arteries (61.9% and 33.3%, respectively), it is important to note the relatively high prevalence of feeding arteries arising from branches of the meningohypophyseal trunk and the inferolateral trunk of the internal carotid artery (38.1%). In group C, the posterior meningeal artery was the most prevalent feeding artery (77.8%).
In terms of target vessels for Onyx embolization, in both group A and B location, the most frequently accessed vessel was the MMA, followed by the OA. In group C, the MMAs were the most frequently catheterized vessel, followed by the posterior meningeal artery. In most procedures, a simple Onyx injection technique was performed using DMSO compatible microcatheters (110 procedures, 92.4%), followed by the balloon assisted technique through a balloon microcatheter, specifically, the Scepter XC Balloon catheter (MicroVention Terumo, Tustin, CA, USA) (7 procedures, 5.9%), and the pressure cooker technique [4], (2 procedures, 2.5%). The latter two adjunctive techniques were utilized when the OAs was selected as the target vessel.
Angiographic outcomes
The immediate angiogram after transarterial Onyx embolization revealed complete fistula obliteration in a total of 41 out of 68 dAVFs (60.3%), with incomplete obliteration in the remainder. Incompletely obliterated lesions included Borden type I lesions in 9 patients, Borden type II in 4, and type III in 14 (13.2%, 5.9% and 20.6%, respectively). Short-term radiographic follow-up data was available in all patients with immediate post-procedure fistula obliteration at a median of 3.9 months (range, 1-18). The most common modality for follow-up was DSA, performed in 37 of 41 dAVFs (90.2%), followed by MRI/A in 9.6% of the dAVFs. Forty (97.6%) of the 41 patients had persistent occlusion of their dAVF. One recurrence of the Borden type III dAVF was observed at 14 months posttreatment without neurological worsening. The patient achieved complete obliteration following an additional transarterial Onyx embolization through the MMA, with a durable occlusion noted until last follow-up at 42 months.
Out of the 27 patients with a residual dAVF, 25 had available radiographic follow-up data, which occurred at a median follow-up of 3.0 months (range, 1-14). Of these, a spontaneous occlusion was observed in 2 patients that had a residual Borden type II (8%), at 3 and 5 months after initial treatment.
Finally, complete fistula obliteration was achieved in a total of 43 (65.2%) of the 66 dAVFs and in 42 (75.0%) of 56 dAVFs with cortical venous reflux (CVR) following transarterial Onyx embolization, at an overall median follow-up of 3.8 months (range, 1-18). A detailed description of angiographic outcomes on follow-up per dAVF location is shown in Table 3.
Clinical outcomes
Clinical follow-up data was available in 66 of 68 dAVFs (97.1%), at a median follow-up of 10.4 months (range, 1.3-138 months). Procedure-related complication occurred in 5 patients (7.3%), including microwire perforation of two MMA and one OA branches, which was subsequently occluded with Onyx without any morbidity. Two patients experienced persistent morbidities (2.9%). One group A patient with a pre-treatment Borden type I dAVF developed cerebellar ischemia due to Onyx migration into the petrosal vein through the superior petrosal sinus (final mRS 1 at 16 months). The other patient, with a group B Borden type III dAVF, developed a middle cerebral artery occlusion secondary to thrombus embolization from a guide catheter in the internal carotid artery, which was successfully recanalized during the procedure (final mRS 2 at 32 month). There were no procedure-related mortalities, and none of the patients developed new neurological symptoms, including cranial nerve palsy, during the follow-up period.
Predictors of a successful fistula obliteration following transarterial Onyx embolization and sub-group analysis
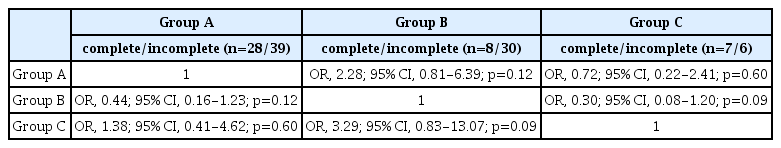
The results of the univariate logistic regression analysis evaluating for potential predictors of a complete fistula obliteration are shown in Table 4. Of these factors, the use of the MMA had a significantly higher chance of complete fistula obliteration (OR), 2.43; 95% CI, 1.12-5.27; p=0.024). The relationship between obliteration and Borden type III dAVFs was found to be marginally significant (OR, 3.05; 95% CI, 0.98-9.49; p=0.054). There was no statistical difference in obliteration status between the three location groups (Table 5).

Univariate analysis of factors for complete fistula obliteration on last follow-up after transarterial Onyx embolization
The multivariable logistic regression model was built by categorizing Borden class (III vs I and II) and MMAs access (MMA vs non-MMA), as mentioned in the methods section. MMA access remained a significant independent predictive factor of complete fistula obliteration (OR, 2.32; 95% CI, 1.06-5.06; p=0.034) (Table 6). To further investigate the effect of pre-treatment Borden type on angiographic outcomes, we performed a subgroup analysis of procedures that used the MMA as a target for Onyx embolization (61 procedures). This showed that group A dAVFs and pre-treatment Borden type III classification were both independent predictive factors of achieving a complete fistula obliteration through the MMA (OR, 5.72, 95% CI, 1.89-17.33, p=0.002; OR, 3.13, 95% CI, 1.05-9.35, p=0.041, respectively) (Table 7).

Multivariate logistic regression analysis of the factors for complete fistula obliteration on last follow-up
Management of residual dAVFs following transarterial Onyx embolization
Of 27 patients with an incompletely obliterated dAVF, 25 had available follow-up data. Of this subset of partially treated dAVFs, CVR remained persistent during follow-up in 11 patients (11/23, 47.8%). One Borden type I dAVF was subsequently treated with transvenous embolization and completely obliterated, and the others have been conservatively managed without both angiographic and clinical progression. Of the patients with CVR, a conventional transvenous embolization was performed in 4 patients (36.4%), a transcranial transvenous embolization in 1 (9.1%), surgical interruption of the CVR in 4 (36.4%), and stereotactic radiosurgery in 2 patients (18.2%). As a result, nine (81.8%) of the eleven patients with CVR achieved complete fistula obliteration following adjunctive treatments.
In total, 52 of 66 dAVFs (78.8%) achieved complete obliteration, including patients with immediate obliteration and patients that underwent adjunctive treatment, and this was observed at a median follow-up of 13.5 months (range, 3 months-11.5 year).
Following adjunctive treatment, two patients with faint early venous filling were radiologically followed, and none developed neurological events. One patient with persistent Borden type III dAVF was recommended adjunctive treatment and one patient with Borden type I dAVF was followed without any progression on angiographic assessments. The treatment flow diagram is shown in Fig. 1.
DISCUSSION
We present clinical and angiographic results from two independent cohorts of patients undergoing transarterial Onyx embolization as a first-line treatment of intracranial dAVFs. In the present study, complete fistula obliteration was identified in 65.2% of cases overall and 75.0% of dAVF cases with CVR after transarterial Onyx embolization, at a median follow-up of 3.8 months. Comparatively, previous studies have reported rates of complete fistula obliteration within the range of 47% to 95% at a similar time period [2,3,5,7,9].
Impact of MMA access on successful embolization
This study shows that transarterial Onyx embolization through the MMA is an independent predictive factor of complete fistula obliteration. This finding could be explained by the differences in the angioarchitecture of the MMA compared to that of the other meningeal arteries. The intradural MMA is anchored to the dura and inner skull and it usually follows a long straight course [8], which is likely a key factor that allows for distal catheterizations close to the fistulous pouch. Therefore, sufficient Onyx penetration through the fistulous pouch and into the draining vein is more likely to occur (Fig. 2). Additionally, the long intradural course may also allow the generation of a firm Onyx plug, creating additional support for Onyx penetration. Furthermore, even though the MMA was not a main feeding artery, its relatively small caliber often enabled a wedge position of the microcatheter and complete obliteration (Fig. 3).
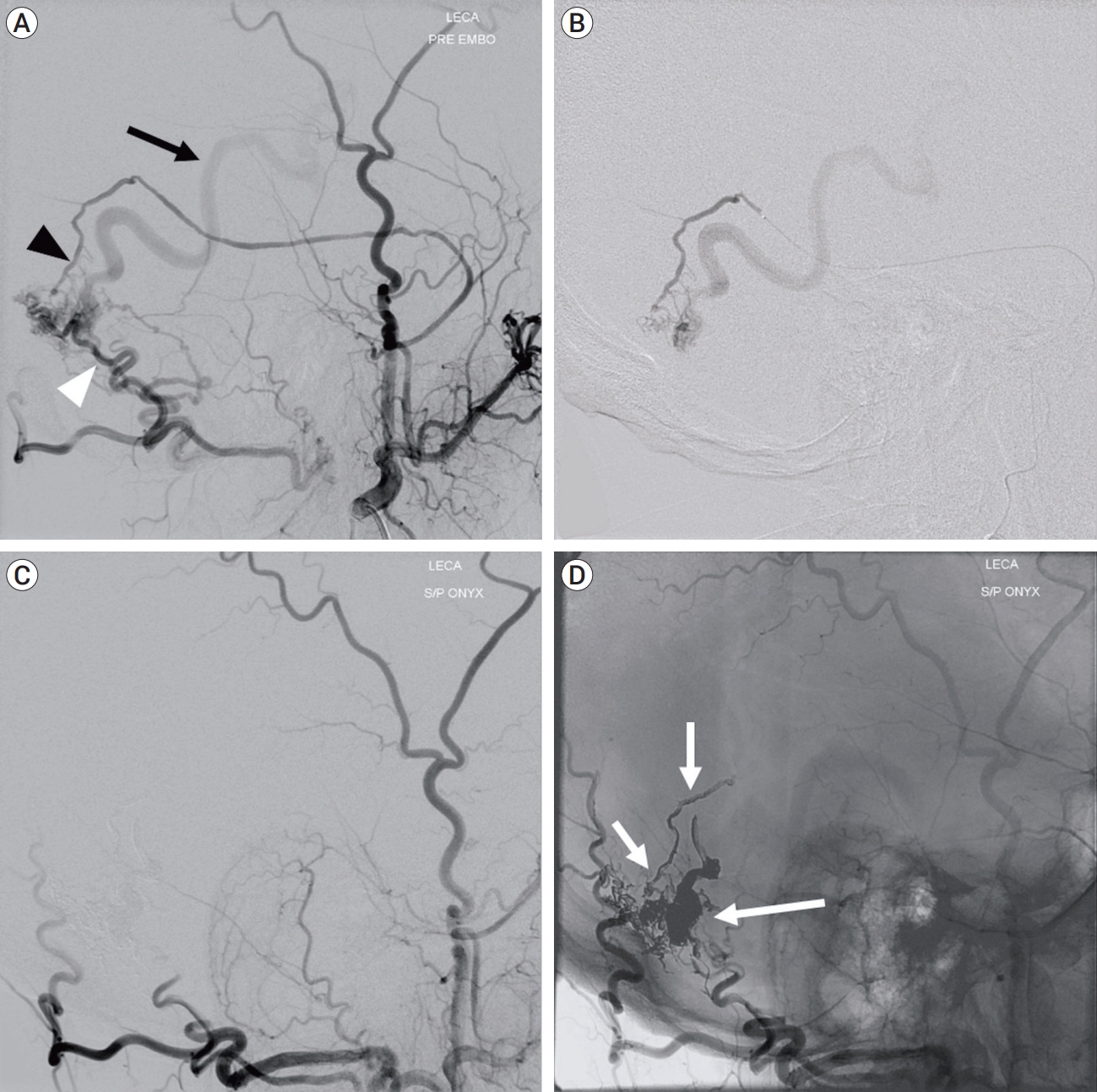
Lateral view of left external carotid artery angiogram showing the dAVF at the isolated transverse sinus fed by the posterior convexity branch of the middle meningeal artery (MMA) (black arrowhead) and transosseous branch of the occipital artery (white arrowhead), and drainage into the dilated cortical vein (black arrow) (A). Selective angiogram of the MMA showing a wedged microcatheter position (B). Left external carotid artery angiogram obtained after embolization confirming complete dAVF obliteration (C). Non-subtracted image showing Onyx cast following embolization (white arrows) (D). dAVF, dural arteriovenous fistula
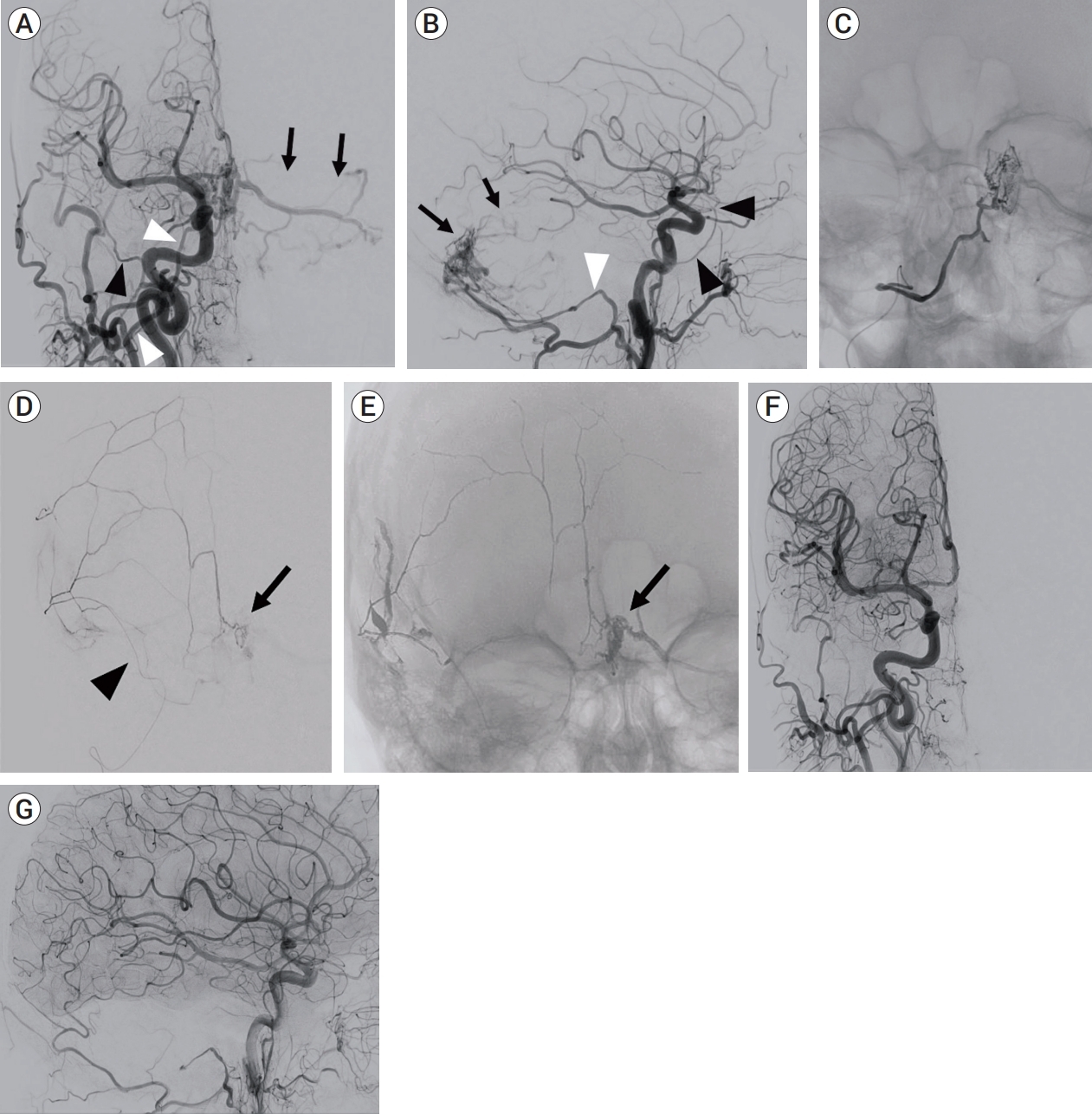
Anteroposterior (A) and lateral (B) view of right common carotid artery angiogram showing a dAVF at the torcula fed by the hypoglossal branches (white arrowheads) of the occipital artery (OA) and draining into left cerebellar cortical veins (black arrows). Note that feeding branches from the middle meningeal artery (MMA) (black arrowheads) are not clearly identified on this injection. Anteroposterior (C) view of selective angiogram of the hypoglossal branch of the OA. Onyx injection was not attempted due to the difficulty of distal catheter navigation. Selective injection from the small caliber MMA showing a wedged microcatheter position (black arrowhead) and contribution to the fistula (black arrow) (D). Non-subtracted image showing Onyx cast penetrating into the fistula pouch (black arrow) (E). Anteroposterior (F) and lateral (G) view of the right common carotid artery angiogram obtained after embolization through the small MMA confirming complete fistula obliteration. dAVF, dural arteriovenous fistula
Interestingly, the occipital arteries were the second most frequently accessed feeding artery but showed a relatively lower rate of achieving complete fistula obliteration. Potential mechanisms underlying this observation include that the occipital arteries usually have a tortuous extracranial course [8], thus preventing distal catheterizations. Second, since these arteries supply intracranial dAVFs through small caliber transosseous branches, the Onyx cast seems to migrate to the branches with larger caliber and lower resistance, such as subcutaneous or muscular branches, rather than penetrating into the fistulous pouch. This rationale could explain the low rates of obliteration in a number of feeding arteries, which supply the dura through transosseous branches. For these arteries, an adjunctive strategy, such as balloon assisted or pressure cooker techniques [4], may allow for improved Onyx penetration into the fistulous pouch.
The posterior meningeal artery was the most frequent feeding artery associated with the dAVF of group C location. Consistent with the results of normal adult cadaveric studies [8], most of the dAVFs in this study, located along the medial cerebellar convexity, were supplied by the posterior meningeal arteries (78%). However, the likelihood of complete fistula obliteration was not as high as that observed in lesions treated through the MMA, especially for the dAVFs of group A location, although this did not achieve statistical significance. This might in part be due to the tortuous intradural course after initial take off of the posterior meningeal artery from the vertebral artery, which precludes distal catheterization and sufficient Onyx penetration into the fistulous pouch, with the additional risk of potential Onyx reflux into the vertebral artery secondary to its short safety margin. The same rationale can apply to the branches of the meningohypophyseal trunk or the inferolateral trunk of the cavernous carotid.
Predictive factors of successful embolization through the MMA
A secondary multivariable analysis demonstrated that achieving a successful complete obliteration through MMA access was significantly associated with group A dAVF location and Borden type III dAVF. The former finding can be explained by the pattern of arterial supply of the dura. The MMA enters the cranium through the foramen spinosum, and gives rise to an anterior, petrous, petrosquamous and convexity branches. Of these, the territories of the convexity branches extend over the entire cerebral convexity and lateral segment of the cerebellar convexity dura and also medially over the falx cerebri, which may explain the higher likelihood of complete fistula obliteration observed in this cohort when performing an transarterial embolization through the MMA in these locations. In contrast, complex patterns of arterial supply to the cranial base and minimal contribution of the MMAs to the medial part of the cerebellar convexity may explain the reduced impact of MMA access on complete fistula obliteration in these locations.
The latter finding of the association between complete obliteration and Borden type III dAVFs which are embolized via the MMA corroborates what has been suggested in the literature. Previous reports have shown a significantly higher rate of a successful transarterial Onyx embolization for dAVF with direct CVR or with CVR through an isolated sinus compared to those with indirect CVR [6,9]. In theory, Borden type I and II dAVFs could require a greater amount of Onyx for achieving a complete obliteration, which carries an underlying risk of both Onyx reflux to dangerous arterial anastomoses and distal Onyx migration into intact venous systems (as seen in one patient of the present series). Therefore, this procedure seems to be more suitable for Borden type III dAVFs.
Strengths and limitations
This study is from a prospective repository of data from patients with dAVFs and the retrospective design of our study is subject to the inherent biases of such a design. The inclusion of 2 centers adds to the generalizability of our findings. However, it also introduces heterogeneity in several areas, such as patient management, follow-up protocols, and imaging. Additionally, the prevalence of feeding arteries may be underestimated due to the presence of competitive flow around the fistula; hence, several small feeders might not be opacified initially but become apparent after obliteration of dominant feeders.
CONCLUSIONS
MMA access is an independent predictive factor of complete obliteration following transarterial Onyx embolization for intracranial dAVFs. Dural AVF located in the supratentorial and lateral cerebellar convexity dura, and Borden type III lesions, were independent predictive factors of complete obliteration through the MMA. Such predictive factors may be useful in selecting arterial access routes for dAVF embolization.
Notes
Disclosure
The authors have no personal financial, or institutional interest in any of the drugs, materials, or devices described in this article.