Use of covered stent (CGuard) in the treatment of post-traumatic internal carotid artery pseudoaneurysm
Article information
Abstract
Post-traumatic internal carotid artery pseudoaneurysm (ICA PSA) is a rare occurrence with high mortality rates, and with the advent of endovascular therapy, its treatment has shown drastic improvement in clinical as well as radiological outcomes. Here we are describing our experience with the CGuard embolic protection system (InspireMD, Tel Aviv, Israel) for the treatment of post-traumatic left ICA PSA in a 49-year-old male. New improved biomechanics and navigability have proven it to be a safe and efficient treatment modality for ICA PSA. However, a multicentric large-scale randomized trial is recommended to support this modality.
INTRODUCTION
Traumatic neck injuries account for 1-3% of all major trauma [6]. Of this, 1% develop internal carotid artery pseudoaneurysm (ICA PSA) [8]. Pseudoaneurysms (PSA) can develop within a few hours to several years after the initial trauma. PSA, unlike true aneurysms, lack all three layers of the arterial wall, i.e., intima, media, and adventitia. It is only covered with an adventitial layer making it prone to rupture and leading to catastrophic consequences. Thus ICA PSA demands urgent intervention. Treatment modalities range from conservative anticoagulant therapy, surgical ligation, and bypass to newer endovascular treatment with coils and stents. We here present a case of post-traumatic left ICA PSA treated with a covered stent, CGuard.
CASE DESCRIPTION
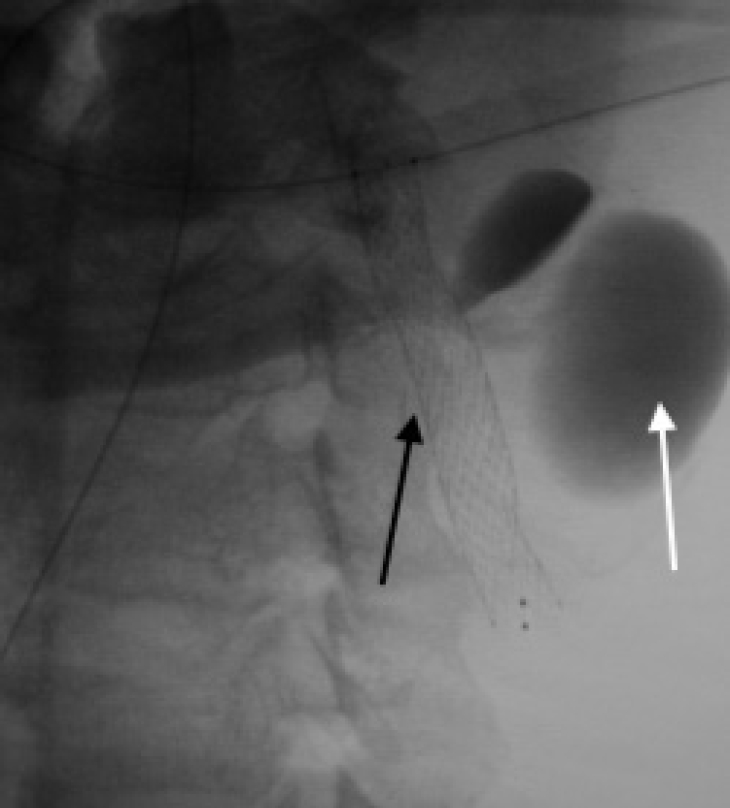
A 49-year-old male presented with a history of progressively increasing pulsatile neck swelling on the left side for the last 1 year. He had a history of neck trauma 5 years back. No neurological deficit was seen on examination. Computed tomography (CT) angiography and digital subtraction angiography (DSA) studies of neck vessels showed a defect in the lateral wall of the left proximal extracranial part of the internal carotid artery with a broad neck PSA measuring 31.0 mm×22.7 mm×20.6 mm (Figs. 1, 2). It was located 27 mm distal to the bifurcation of the common carotid artery with kinking of the ICA just proximal to PSA (Fig. 2). The patient was given Ecosprin 150 mg/day and Prasugrel 10 mg/day for 3 consecutive days prior to the procedure, and all procedures were done under general anesthesia. Intra-arterial pressure monitoring was used to maintain optimum hemodynamics. An 8-French 13 cm femoral access sheath (Cook Medical, Bloomington, IN, USA) was used for groin access on the right side. An 8-French 90 cm Neuron MAX long sheath (Penumbra, Alameda, CA, USA) was used and placed near the aortic arch. A 5-French 130 cm Neuron Select guide catheter (Penumbra, Alameda, CA, USA) was used to access the left ICA. This helped to straighten the ICA and resolve the kink (Fig. 3A). A Synchro 200 cm micro-guide wire (Stryker Neurovascular, Fremont, CA, USA) was used to cross the PSA and was anchored in the petrous part of the left ICA. CGuard 6.0 mm×40 mm self-expandable open-cell covered stent (InspireMD, Tel Aviv, Israel) was deployed across the neck of the PSA successfully. Post-procedure DSA showed stasis in PSA and patent parent artery (Fig. 3B). Stent graft could be seen in the stentogram done after the procedure (Fig. 4). No complications occurred during the procedure. CT angiography of neck vessels with 3D reconstruction was done, which confirmed the complete exclusion of the PSA and patent parent artery (Fig. 5). The postoperative course was uneventful, and the patient was discharged on dual anti-platelets therapy for follow-up after 3 months. Informed consent was taken from the patient to use his information and imaging for this paper.

Pre-op CT angiography of neck vessels, (A) axial view and (B) coronal view with, (C) 3D reconstruction showing pseudoaneurysm (PSA) in extracranial part of the left internal carotid artery (ICA) shown with black arrows. CT, computed tomography

Pre-op DSA of neck vessels, (A) anteroposterior view and (B) lateral view with, (C) 3D reconstruction showing pseudoaneurysm (PSA) in extracranial part of the left internal carotid artery (ICA) with kinking (black arrow) of ICA seen distal to bifurcation of common carotid artery (CCA) with dimensions of the PSA. DSA, digital subtraction angiography
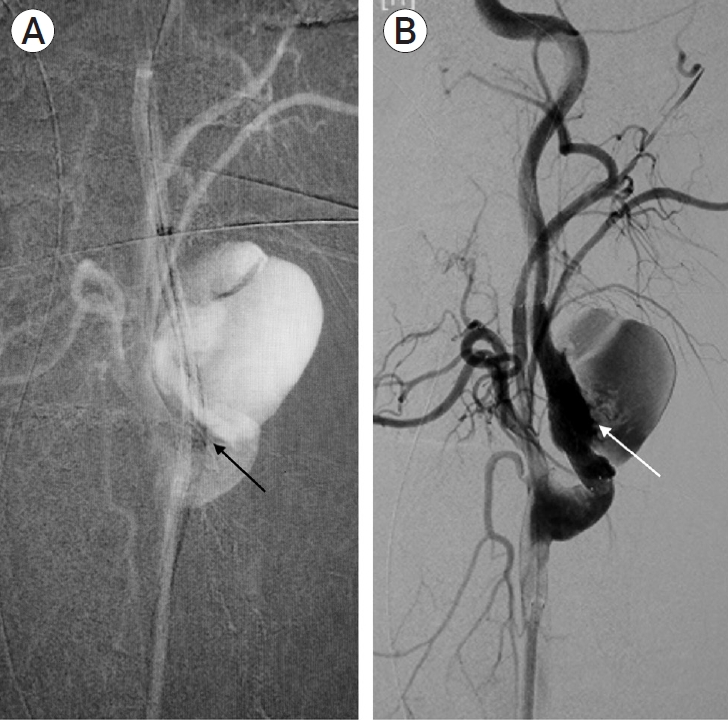
Intra-op DSA with (A) straightening of the ICA (black arrow) by Neuron Select guide catheter (Penumbra, Alameda, CA, USA) in-situ and, after (B) deployment of CGuard Stent (InspireMD, Tel Aviv, Israel) across pseudoaneurysm (white arrow). DSA, digital subtraction angiography; ICA, internal carotid artery
DISCUSSION
Traumatic neck injuries account for 1-3% of all major trauma [6]. Out of this, 1% develop ICA PSA [8]. Biffl Scale for Blunt Cerebrovascular Injury (BCVI) classified BCVI into five types, i.e., I-V1. PSA formation is graded as type III injury. Other causes of PSA are arterial dissection, vasculitis (e.g., Bechet’s disease), infections (e.g., Mycotic), and iatrogenic (e.g., Carotid endarterectomy). PSA can develop within a few hours to several years after the initial trauma. Diagnosis is established with Doppler ultrasound and CT angiography of neck vessels.
PSA, unlike true aneurysms, lack all three layers of the arterial wall, i.e., intima, media, and adventitia. It is only covered with an adventitial layer making it prone to rupture and leading to catastrophic consequences. Also, traumatic ICA PSA is associated with acute cerebral ischemia in 12.2% of cases [7] and is a leading cause of stroke in young (16-45 years of age) [2]. Mortality of traumatic ICA PSA and neck injuries overall varies from 20-30%. Thus, ICA PSA demands urgent intervention.
Treatment modalities range from conservative anticoagulant therapy, surgical ligation, and bypass to endovascular treatment with coils and stents. Initially, PSA was conservatively managed with observation and anticoagulant therapy. It was found that anticoagulant therapy alone has paradoxical effects with no improvement rather it increased wall hemorrhage. It was also contraindicated in patients with multiple trauma or severe injury. So conservative therapy of PSA was soon discarded. Surgical ligation of PSA in accessible lesions was performed with high postoperative morbidity and mortality. Occlusion intolerance and retrograde flow were some of the complications of this modality. Carotid bypasses were tried with similar difficulties as with ligation. Difficult exposure and risk of stroke were seen in almost 9% of the patients [3]. The development of endovascular procedures such as coiling and stent placement has emerged as a safe and efficient treatment of PSA.
The new endovascular modality of covered stenting reduces leakage of pseudoaneurysm and promotes intra-aneurysmal thrombosis. Wang et al., in their study of five patients of post-traumatic carotid PSA being treated with a covered stent, found satisfactory long-term outcomes about the complete exclusion of PSA and patency of parent artery [9]. However, this study was the first of its kind and included a small volume of cases. Ajay et al. evaluated the role and efficacy of the endovascular intervention on twenty patients with ICA PSA. They found no major complications in seven patients undergoing covered stenting [5]. The limitation of this study was the small sample size. Hoppe et al., in their study on twenty-five patients, found technical success in 100% of cases but with significant complication rates [4].
Recent development in endovascular treatment led to the introduction of the CGuard Carotid Embolic Protection System (CGuard Carotid EPS) by InspireMD with a self-expandable covered stent. It is an open-cell design with the inner layer of bare metal made from nitinol covered by an outer layer of micron-level mesh (MicroNet) made from polyethylene terephthalate (PET) fibers attached to proximal and distal edges of the stent. CGuard is available as 6-10 mm×20-60 mm (diameter×length) sizes, and when fully expanded, the pore size is 150-180 microns. Due to this small size of pores of MicroNet mesh, there is a decreased plaque rupture and prolapse formation leading to low thromboembolic risks and cerebral stroke in both peri-procedural and post-procedural follow-up. Though clinical data supporting this is based on small study groups and single-centric. The diameter of the struts of CGuard is 20-25 microns which is comparable to that of flow diverters (FD), due to which there is an immediate diversion of flow and stasis in PSA as seen in our case. The bending stiffness of CGuard on its delivery catheter is 601 N·mm2, which is amongst the stiffest, which helps navigate through kinked arteries. The stiffness of the expanded CGuard is 63.1 N·mm2 that is lower than other double-layered stents. CGuard has a higher radial force but a lower collapse pressure providing better support to the diseased section of the artery. The company also claims to have provided “SmartFit” technology which eliminates the need for a tapered version. Christian et al., in their study, found CGuard to prevent postprocedural embolic events [10]. He also found that the stent is easy to use and safe to implant as it does not foreshorten, and its structure adapts well to changes in diameter and direction of tortuous vascular anatomies.
CONCLUSIONS
Post-traumatic ICA PSA is a rare occurrence but with high mortality rates. Previous treatment modalities have shown poor postoperative and long-term results. With the advent of endovascular therapy and its recent advances, the treatment of ICA PSA has shown drastic improvement in clinical and radiological outcomes. The introduction of novel CGuard for the treatment of post-traumatic ICA PSA has shown to be a safer and better alternative than other available treatment modalities. Previous clinical research supports the use of CGuard in the treatment of ICA PSA, but further investigations in the form of multicentric large-scale randomized trials are recommended.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.