|
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 24(1); 2022 > Article |
|
Abstract
Fig.┬Ā1.
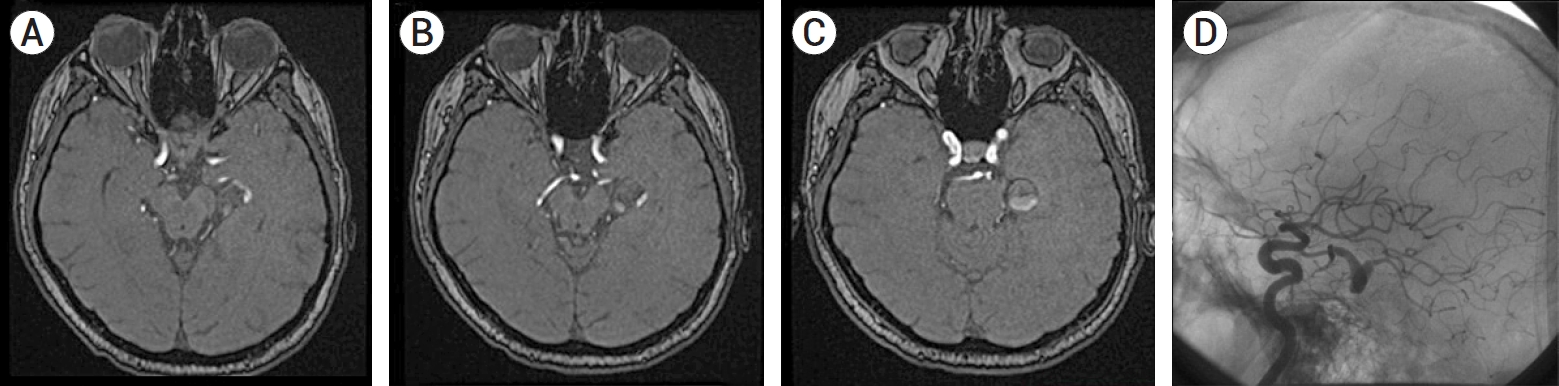
Fig.┬Ā2.
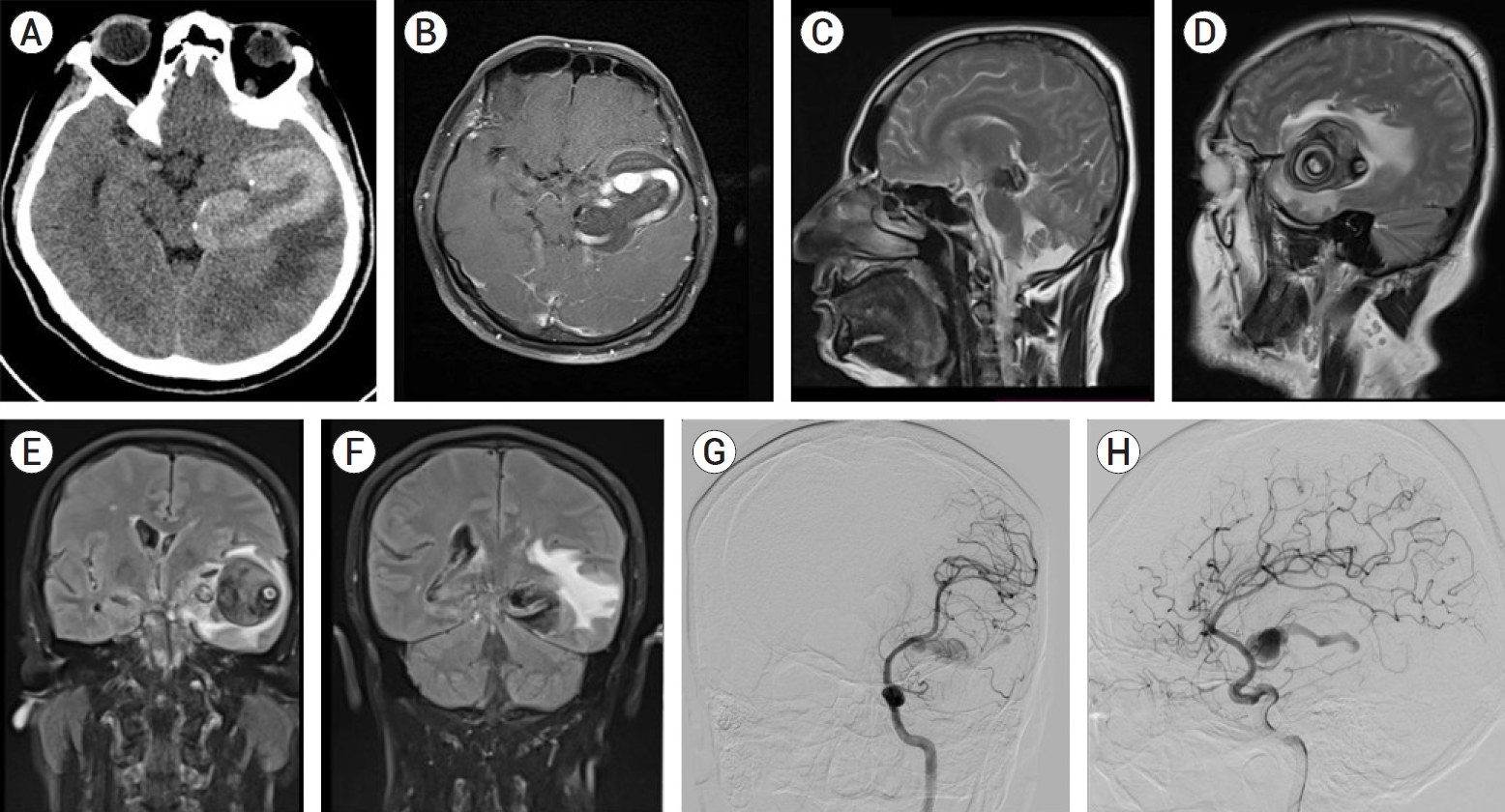
Fig.┬Ā3.

Fig.┬Ā4.

Table┬Ā1.
| Authors | Patient number | Aneurysm location | Treatment type |
|---|---|---|---|
| Mizuno et al. [12] 2021 | 1 | BA | EVT |
| Dao et al. [6] 2021 | 22 | 12 PCA | EVT |
| 7 MCA | |||
| 3 ACA | |||
| Mura et al. [13] 2021 | 1 | MCA | Bypass, clipping |
| Tao et al. [18] 2020 | 1 | ACA | Bypass, aneurysm resection |
| Deng and Feng [7] 2019 | 1 | ICA | EVT |
| Li et al. [11] 2019 | 3 | N/A | N/A |
| Lan et al. [10] 2018 | 1 | MCA | Aneurysmectomy, bypass, proximal occlusion and vascular reconstruction |
| Benet et al. [3] 2017 | 1 | ACA | Bypass, clip occlusion |
| Kandemirli et al. [9] 2018 | 15 | 7 MCA | EVT |
| 4 PCA | |||
| 2 ICA | |||
| 1 ACA | |||
| 1 BA | |||
| Yoshimura et al. [19] 1994 | 1 | 1 ICA and 1 MCA | Bypass and proximal occlusion |
| All patients in the review of Kandemirli et al. [9] 2018 | 100 | 48 MCA | Various types |
| 17 PCA | |||
| 14 (VA, VBJ, BA, PCoA, PICA or SCA) | |||
| 13 ICA | |||
| 9 ACA |
GSA, giant serpentine aneurysm; BA, basilar artery; EVT, endovascular therapy; PCA, posterior cerebral artery; MCA, middle cerebral artery; ACA, anterior cerebral artery; ICA, internal carotid artery; N/A, not available; VA, vertebral artery; VBJ, vertebrobasilar junction; PCoA, posterior communicating artery; PICA, posterior inferior cerebellar artery; SCA, superior cerebellar artery.
REFERENCES
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 2,703 View
- 77 Download
- ORCID iDs
-
Fatih Yakar

https://orcid.org/0000-0001-7414-3766 - Related articles
-
Endovascular treatment of intracranial aneurysms: Past and present
Giant cerebellar cavernous malformation in children: A case report and literature review
Ruptured mirror DACA aneurysm: A rare case report and review of literature2023 September;25(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print