Readmission into intensive care unit in patients with aneurysmal subarachnoid hemorrhage
Article information
Abstract
Objective
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating cerebrovascular event; patients are routinely admitted to the intensive care unit (ICU) for initial management. Because complications may be delayed, unplanned ICU readmissions can occur. Therefore, in this study we evaluate the rate of and factors associated with readmission after aSAH and identify if readmission is associated with poor clinical outcomes.
Methods
We retrospectively reviewed the medical records of all patients receiving surgical or endovascular treatment for aSAH and admitted to the ICU between January 2008 and December 2019. We categorized patients by readmission and analyzed their clinical parameters.
Results
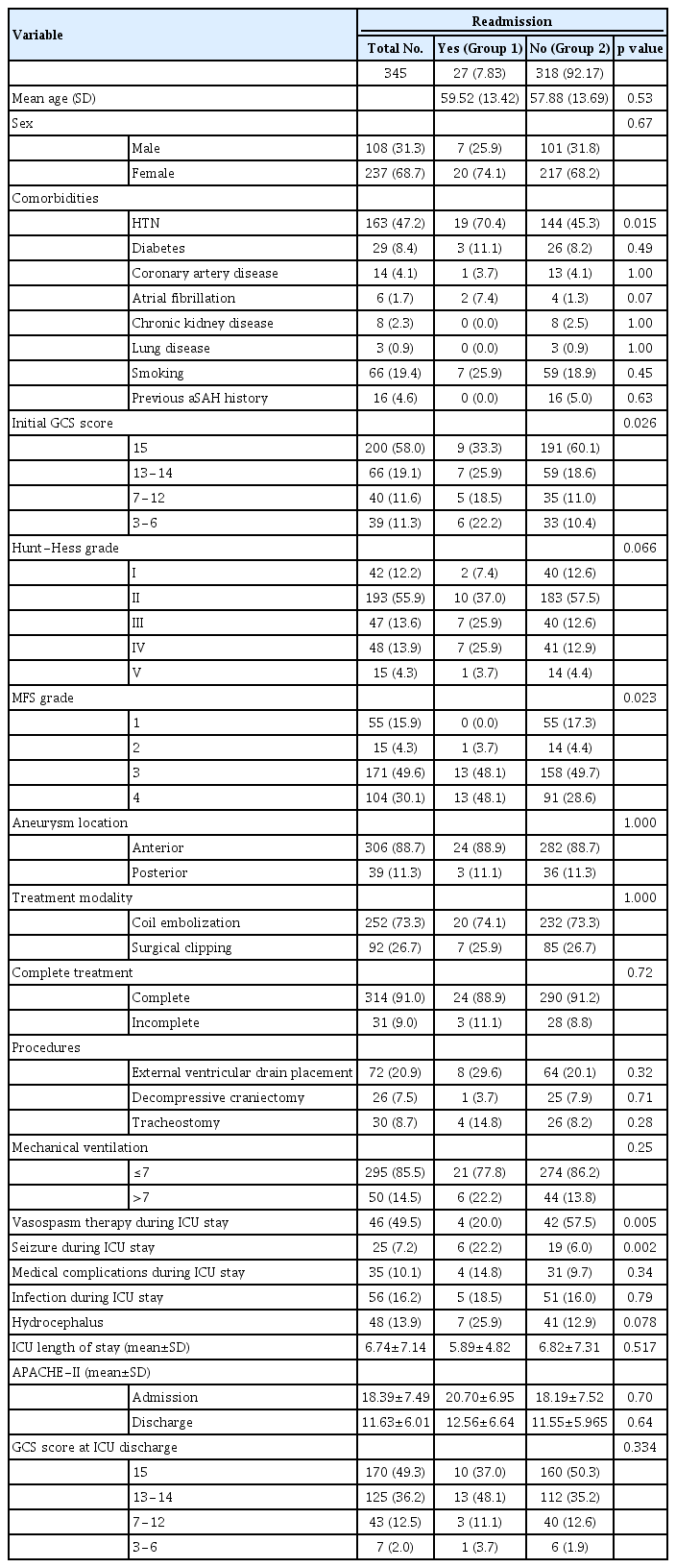
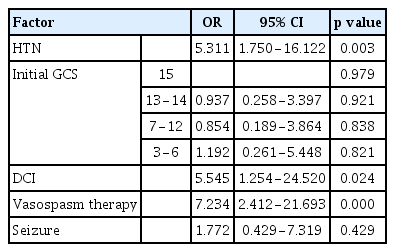
Of the 345 patients who transferred to ward-level care after an initial ICU stay (Group 2), 27 (7.3%) were readmitted to the ICU (Group 1). History of hypertension (HTN), initial Glasgow Coma Scale (GCS) score, modified Fisher grade, and vasospasm therapy during first ICU stay were significantly different between the groups. The most common reason for readmission was delayed cerebral ischemia (DCI; 70.3%; OR 5.545; 95% CI 1.25−24.52; p=0.024). Comorbid HTN (OR 5.311; 95% CI 1.75−16.12; p=0.03) and vasospasm therapy during first ICU stay (OR 7.234; 95% CI 2.41−21.7; p<0.01) also were associated with readmission. Readmitted patients had longer hospital stay and lower GCS scores at discharge (p<0.01).
Conclusions
DCI was the most common cause of ICU readmission in patients with aSAH. Readmission may indicate clinical deterioration, and patients who are at a high risk for DCI should be monitored to prevent readmission.
INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating cerebrovascular event, and patients with aSAH are routinely admitted to the intensive care unit (ICU) during initial management [2]. Even after surviving near-fatal early complications such as rebleeding, secondary neurological complications such as delayed cerebral ischemia (DCI) or hydrocephalus may occur. Moreover, aSAH predisposes patients to medical complications that may cause poor clinical outcomes [14]. As complications may occur after the initial aneurysm rupture, some patients may experience an unplanned readmission to the ICU. Therefore, in this study we evaluated the rate and factors associated with readmission after aSAH and identified whether readmission is associated with poor clinical outcomes.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of all patients with aSAH receiving surgical or endovascular treatment for aneurysm obliteration and admitted to the ICU at our facility between January 2008 and December 2019. We identified patients who were readmitted to the ICU having previously been discharged to ward-level care, excluding those patients who died or were discharged during index admission. We collected data on patient age; sex; history of hypertension (HTN), diabetes mellitus, or other comorbidities; Hunt−Hess and Modified Fisher Grading Scale (MFS) levels; Glasgow Coma Scale (GCS) scores at admission and discharge; modified Rankin Scale (mRS) at discharge; length of initial and subsequent ICU stays; indications for readmission; and mortality rate.
We categorized patients by ICU readmission and statistically analyzed their clinical parameters at ICU discharge using the chi-squared, Mann−Whitney U, or Fisher’s exact tests, as well as Cox regression to compare the groups. We performed the descriptive statistical analysis using SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 369 patients with aSAH were admitted to our ICU (we excluded 24 patients who had died or transferred to hospice care during their first ICU stay). Of the 345 patients who transferred into ward-level care after their initial ICU stay, 27 patients were readmitted to the ICU, a rate of 7.83%.
Baseline patient characteristics
Table 1 shows patient demographic characteristics. Group 1 includes patients who were readmitted to the ICU after ICU discharge, and Group 2 includes those not readmitted. The mean age of Group 1 was 59.52 years, and women comprised 74.1% of this group. Age (p=0.53) and sex (p=0.67) did not differ between the groups. The most common comorbidity of the total population was HTN history (47.2%); we found no significant differences in comorbidities between the groups except for HTN.
GCS scores were grouped as 15, 13−14, 7−12, and 3−6, with reference to World Federation of Neurosurgical Societies grade. GCS score 15 was most prevalent in Group 2 (60.1%); GCS scores tended to be significantly lower in Group 1 (p<0.05).
The groups had no differences in Hunt−Hess grade, and Grade II was most prevalent in both groups (Groups 1 vs. 2: 37.0% vs. 57.5%; p=0.066). In contrast, the majority of patients in Group 1 had modified MFS Grades 3 and 4 (96.2% vs. 78.3%; p=0.023), which was significantly different from those in Group 2. No significant differences existed in location or incomplete obliteration of the ruptured aneurysm or in treatment modality.
External ventricular drain placement, decompressive craniectomy, or tracheostomy were not different between the groups, but vasospasm therapy following symptomatic vasospasm during first ICU stay was significantly higher in Group 1 (p=0.005), as was incidence of seizure (p=0.002). Medical complications, infection rate, and mean length of initial ICU stay were not significantly different between the groups.
Causes of readmission
Neurological deterioration as a cause for readmission accounted for 70.4% (n=19) of the total readmissions, and DCI was the most common neurological complication (55.6%). A total of 18.5% (n=5) of the patients had infection, 7.4% (n=2) of whom had septic shock (Table 2). Other causes included hyponatremia, pulmonary edema, and pulmonary thromboembolism.
Factors associated with readmission
We analyzed co-existing HTN, initial GCS score at time of admission, history of vasospasm therapy and seizure during initial ICU stay, and development of DCI, as these factors showed significant differences between the two groups. Co-existing HTN (OR 5.311; 95% CI 1.75−16.12; p=0.03), history of vasospasm therapy during ICU stay (OR 7.234; 95% CI 2.41−21.7; p<0.01) and DCI (OR 5.545; 95% CI 1.25−24.52; p=0.024) were associated with readmission (Table 3). Patients receiving vasospasm therapy had 7 times the readmission rate.
Outcomes
Mean length of the entire hospital stay was longer (33.7 days) in Group 1 than in Group 2 (20.9 days; p<0.01) (Table 4). GCS score at first ICU discharge was not significantly different between the groups, but GCS score at hospital discharge was significantly different, especially a GCS score of <13 (Groups 1 vs. 2: 23.2% vs. 11.0%; p<0.01).
The scores on the mRS, which indicates functional status, also revealed significant differences. Compared with the readmission group, there was a difference of 0–2 mRS in the nonreadmission group (the symptoms were mild in the group, enabling independent living), 70% versus 40.7% (p=0.001).
DISCUSSION
Unplanned readmission to ICU represents clinical deterioration and is accompanied by longer ICU stay and in-hospital mortality [7]. Given that patients with aSAH may have delayed presentation of post-aSAH complications, including DCI and other neurological impairments or functional disabilities, ICU readmission may have clinical implications.
Although several studies have identified risk factors for hospital readmission for patients with aSAH [4,11], few studies have evaluated ICU readmission after initial admission. In this study, we focused on evaluating the readmission rate of patients during their first hospital stay and determining the causes of ICU readmission. DCI was the most common cause, accounting for 55.6% (n=15) of the patients, a result similar to that in another study [9]. This finding was not surprising, because 79.7% (n=275) of our study population had MFS Grades III or IV.
Previous analyses of patients with aSAH have found that DCI is a hazardous complication leading to delayed neurological deterioration [13]. The increased burden of blood volume in the subarachnoid and intraventricular spaces has been associated with symptomatic vasospasm, cerebral ischemia, and delayed ischemic neurological deficits [3]. However, these do not always indicate poor neurological status (e.g., level of consciousness) requiring intensive care. Therefore, even patients with MFS Grades III or IV may be alert during their initial ICU stay after obliteration of the ruptured aneurysm followed by initial management. Rather, a poor neurological grade is known to correlate with a prolonged ICU stay [15], and patients with Hunt−Hess Grades of <III or IV may require longer monitoring and treatment times in the ICU and thus do not require readmission after discharge to ward-level care.
Ironically, neurological status, represented with GCS score, at admission or ICU discharge, was not related to readmission. Patients at a higher risk of DCI but presenting with favorable and stable neurological status during their first ICU stay, which led to an early ICU discharge, were more likely to be readmitted. ICU discharge criteria included neurological stability with control of seizures and increased intercranial pressure (IICP), stable hemodynamic parameters, and no need for organ support. However, these general ICU criteria did not successfully prevent readmission because of a variety of delayed complications, especially DCI.
Thus, establishing specific discharge criteria for these conditions is challenging. Due to limited resources, retaining patients in the ICU not needing intensive care is difficult, as other patients with a higher need have priority. Moreover, complications associated with prolonged ICU stay (e.g., delirium, post-traumatic stress disorder [PTSD]) must be considered. Therefore, ICU risk−benefit analyses must be conducted to determine the problems of prolonged stay, resource allocation, and stay advantages to quickly cope with neurological deterioration. More personalized ICU admission and discharge criteria are needed.
Detecting DCI often was delayed after transfer to ward-level treatment, leading to a noticeably worse neurological condition at readmission to the ICU than that during the transfer out. Therefore, patients should be monitored closely in an intermediate unit with a higher level of care than ward-level care but with less-intensive care than the ICU. Intermediate units are available in many ICU settings as step-down transfer care [10]. For patients not needing active life-supporting treatment (e.g., mechanical ventilation) or neurological support for IICP or seizure could be monitored in an intermediate unit rather than in the ICU. These patients could avoid the discomfort of invasive monitoring devices and may become less susceptible to delirium or PTSD by their being allowed to ambulate.
Previous research has suggested that providing an intermediate unit may avoid patient readmission to the ICU and improve overall ICU utilization [8,12]. Our center’s intermediate unit for neurosurgical patients has been in operation since July 2019. Since the introduction of the intermediate unit, early detection of neurological deterioration in patients has been possible, and these patients have had the advantages of reversing their neurological condition, even when they were readmitted to the ICU. However, given the short time in operation, further analysis of the effects of this unit is needed.
We found that HTN is a potential risk factor for ICU readmission. Although controversy remains for this finding, several studies have demonstrated a significant association between pre-existing HTN and DCI [6]. The brain of a patient with HTN may be more prone to ischemia. In chronic HTN, the autoregulatory curve is shifted to the right so that small blood pressure reduction can lead to cerebral ischemia.5 Therefore, evaluating the status of cerebral autoregulation in each patient and maintaining the optimal range of blood pressure can prevent further ischemic damage during the acute period. However, currently no way exists to monitor the status of cerebral autoregulation noninvasively in real time, and it is difficult to assess this issue in ward or intermediate-unit settings. An appropriate monitoring method can help prevent and manage DCI.
Another important cause of readmission was infection, which is common after aSAH, and severe hospital-acquired infections (e.g., pneumonia, bloodstream) are known risk factors for prolonged hospital stay and poor clinical outcomes.1 In our study, infection was the second most common cause of readmission. Among five patients (18.5%), we identified septic shock and aspiration pneumonia in two patients each and central line–associated blood stream infection in one patient. These severe, potentially life-threatening infections are strong indicators for ICU readmission and also can independently contribute to worsening outcomes. Given that frequent functional impairment may have significant effects on the incidence of infection, close monitoring of these symptoms is necessary.
This study has several limitations. First, we studied a small number of patients from a single tertiary medical center. Second, we based our evaluations on a retrospective chart review, which likely did not capture subtle elements such as the availability of ICU beds. Finally, although designated clinicians were in charge of patients’ clinical judgment, the decision to discharge from or readmit to ICU can vary. In addition, the practice patterns of our institution may not match those at other centers, so these results cannot be generalized to patients with aSAH treated at other academic or community hospitals. Therefore, follow-up studies are needed.
CONCLUSIONS
aSAH patient readmission to ICU can indicate deterioration in clinical status. More consideration should be given to discharging patients who are at high risk of DCI, even after surviving initial vasospasm therapy, as these patients are prone to readmission.
Notes
Disclosure
The authors report no conflicts of interest concerning the materials or methods used in this study or the findings specified in this article.