Endovascular treatment of residual or recurrent intracranial aneurysms after surgical clipping
Article information
Abstract
Objective
Total aneurysm occlusion is crucial for the prevention of rebleeding of a ruptured aneurysm or to avoid rupture of an unruptured lesion. Both surgical and endovascular embolization fail to achieve complete aneurysm occlusion in all the cases. The objective of the study was to establish the safety and efficacy of endovascular treatment for previously clipped residual or recurrent aneurysms.
Methods
This was an observational, retrospective study of patients harboring incompletely occluded intracranial aneurysms after clipping who underwent endovascular treatment. Patients were treated using 4 different techniques: (1) simple coiling, (2) balloon remodeling, (3) stent-assisted coiling, and (4) flow diversion. Analyses were performed to identify predictors of total aneurysm occlusion, recanalization and complications.
Results
Between May 2010 and September 2018, 70 patients harboring incompletely occluded intracranial aneurysms after clipping met the inclusion criteria in 5 centers. The mean residual aneurysm size was 7.5 mm. Fifty-nine aneurysms were unruptured. Total aneurysm occlusion was achieved in 75.3% of the aneurysms after 1 year. All aneurysms treated with flow diversion revealed complete occlusion according to control angiography. Recanalization was observed in 14.5%. Permanent morbidity and mortality occurred in 2.9% and 1.4% of the patients, respectively.
Conclusions
Endovascular treatment of recurrent or residual aneurysms after surgical clipping was safe and efficacious. Flow diversion seems to be associated with better anatomical results. A more rigid study, a larger group of patients, and long-term follow-up are required to provide stronger conclusions about the best approach for residual clipped aneurysms.
INTRODUCTION
Embolization is increasingly used to treat intracranial aneurysms, as endovascular techniques have continued to improve [1,4,24]. The total occlusion of the aneurysms is crucial for the prevention of rebleeding of a ruptured aneurysm or the subsequent growth and to avoid rupture of an unruptured lesion [16]. However, both surgical clipping and endovascular treatment (EVT) fail to achieve complete aneurysm obliteration in all cases [5,7,9,20].
The rates of total occlusion favor patients who undergo clipping compared with those who undergo coiling [23]. However, postoperative aneurysm remnants can occur in up to 18% of patients who undergo angiography after surgery [10,16,28,30]. Even in high-level neurosurgical centers, incomplete obliteration is faced in a significant proportion of clipped aneurysms. The Barrow Ruptured Aneurysm trial revealed that 13% of surgically treated aneurysms were not totally occluded in postoperative radiological control [28]. A similar result was published by a high-skilled Finland group, who achieved complete aneurysm occlusion in 88% of aneurysms [16]. The routine utilization of 3D angiography, which provides excellent image quality and various viewing angles, may explain the increased rate of residual aneurysms detected [13].
The annual risk for rebleeding from aneurysm remnants has been reported as 1.9%. This number is higher than the bleeding risk from unruptured untreated aneurysms [2]. The degree of ruptured aneurysm occlusion after treatment is strongly associated with the risk of rerupture (from 1.1% for complete occlusion to 17.6% for occlusion <70%) [11].
The results of EVT, including retreatment of a residual or recanalized coiled aneurysm, have been extensively studied [22,27]. Surgical treatment of previously embolized aneurysms has also been described in some series [3,26].
Embolization has been successfully performed in patients with residual or recurrent aneurysms after surgical clipping [8,14,19,25]. Although it may represent an excellent alternative to reoperation, the results of EVT in such scenarios must be further investigated.
We present the largest reported case series to date of EVT for previously clipped aneurysms. A multicenter study was conducted to establish the safety and efficacy of the treatment and to identify the predictors of total aneurysm occlusion, recanalization and complications.
MATERIALS AND METHODS
Study design
This was an observational, retrospective, multicenter study conducted in accordance with the Strengthening of the Reporting of Observational Studies in Epidemiology (STROBE) statement and was approved by the ethics board of all institutions, which waived the need for written informed consent from the participants.
The inclusion criteria were as follows: (1) patients of all ages harboring residual or recurrent saccular intracranial aneurysms; (2) previously surgically treated aneurysms; (3) unruptured or ruptured aneurysms; and (4) an attempt to retreat the residual aneurysm by an endovascular approach. The exclusion criteria were as follows: (1) de novo aneurysms; and (2) aneurysms close to a surgical clip but with radiological evidence that the target lesion (clipped) was another adjacent aneurysm.
Patients were referred for EVT after evaluation by the neurosurgical team. In most cases, the aneurysms had been initially treated in other centers. An aneurysm was considered to have a remnant if a treated aneurysm was found to have a residual filling. Reasons for retreatment included: aneurysm remnants presenting with rupture; for unruptured aneurysms, hemorrhage on initial presentation prior to clipping, enlarging remnants, or aneurysm remnants larger than 3 mm in diameter.
Endovascular procedure
Patients were treated using 4 different techniques: (1) simple coiling (SC), (2) balloon assisted coiling (BAC), (3) stent-assisted coiling (SAC), and (4) flow diversion (FD). The treatment strategy was decided on a case-by-case basis.
Ruptured narrow-neck aneurysms were treated by SC, and wide-neck aneurysms were treated with BAC or FD. SAC and FD were not used whenever possible to avoid dual antiplatelet therapy in the acute phase of hemorrhage. One patient was treated with FD once he had an uncoilable aneurysm.
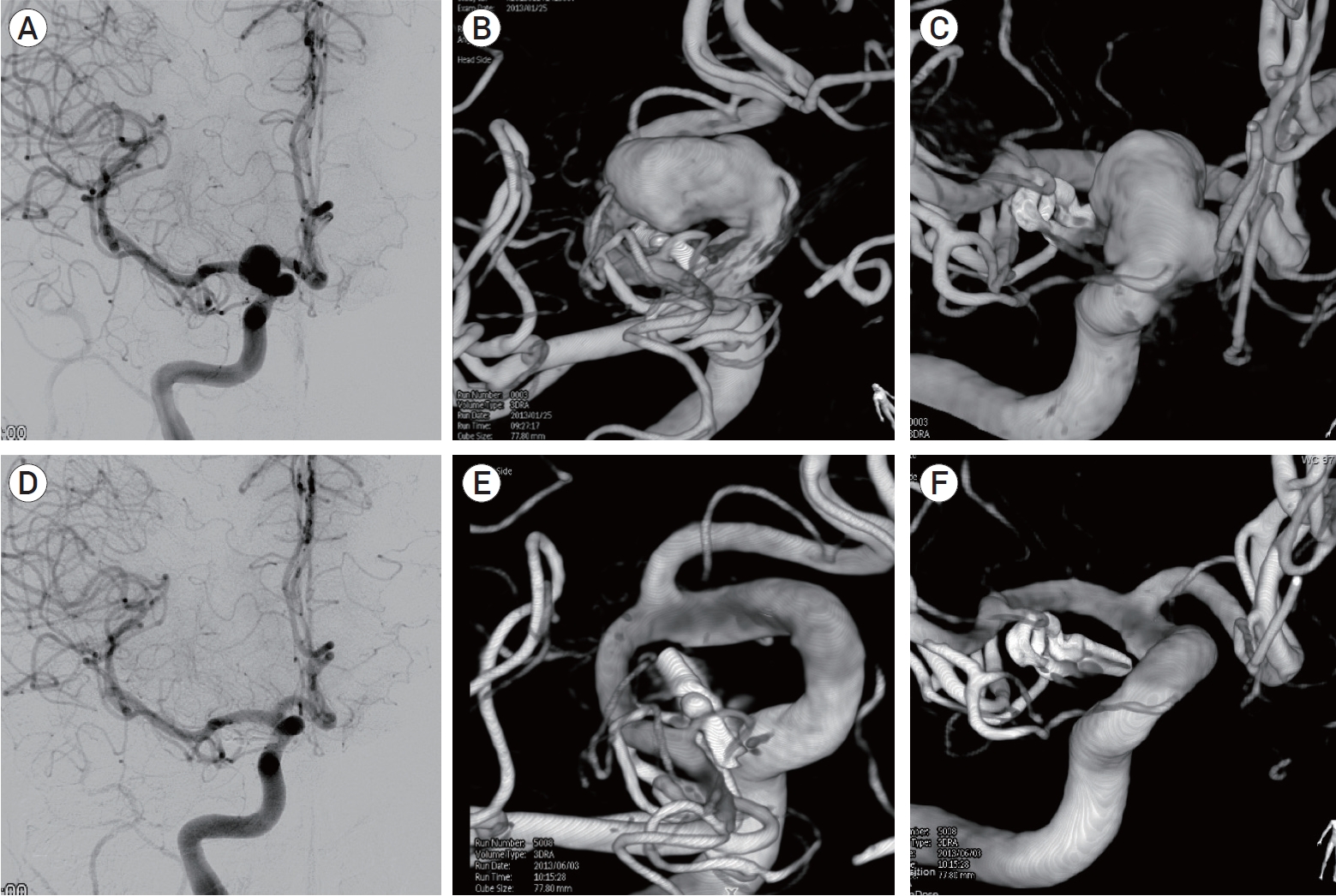
Unruptured aneurysms were managed as follows: narrow-neck aneurysms were preferably treated with SC; wide-neck aneurysms were preferably treated with BAC or SAC if they were located at a bifurcation, and with a FD if they were located at a sidewall (Fig. 1).
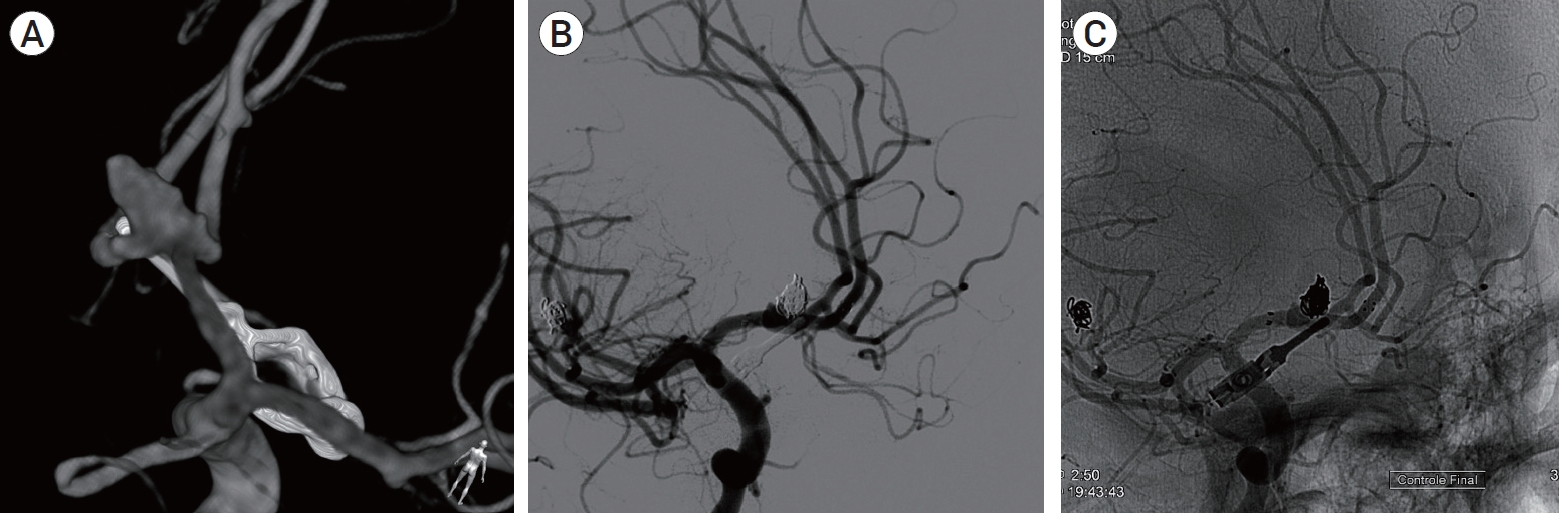
(A) 3D rotational angiography showing a residual anterior communicating artery aneurysm after clipping. (B) Control angiography after stent-assisted coiling. (C) Note the laser-cut stent deployed from A1 to contralateral A2.
Patients with unruptured aneurysms were treated in an elective fashion. They were asked to take aspirin (100 mg) for 5 days prior to treatment. If deployment of a stent or a flow diverter device was planned, clopidogrel (75 mg) was added to the aspirin regimen. For SAC, clopidogrel was given for another 1 month, and for FD, it was given for another 6 months. Aspirin was maintained for 12 months.
For ruptured aneurysms, antiplatelet therapy was not used, except in one case of flow diversion treatment. That patient received a loading dose of aspirin (300 mg) and ticagrelor (180 mg) 3 hours prior to procedure. Ruptured aneurysms were treated on the same day of the diagnosis of incomplete occlusion.
All cases were treated under general anesthesia. Heparin was given in an initial bolus of 5,000 IU and adjusted to achieve and maintain an activated clotting time between 250 and 300 seconds. Anticoagulation was allowed to reverse spontaneously.
Two different coils (Target coil; Stryker, Fremont, CA, USA; or Axium coil; Medtronic, Irvine, CA, USA) and four different balloons (Transform C and SC Balloons; Stryker, Fremont, CA, USA; or Hyperglide and Hyperform Balloons; Medtronic, Irvine, CA, USA) were used. Two intracranial stents (Neuroform stent; Stryker, Fremont, CA, USA; or Solitaire stent; Medtronic, Irvine, CA, USA) and 4 different flow diverter devices (Pipeline; Medtronic, Irvine, CA, USA; Derivo; Acandis, Pforzheim, Germany; Silk; Balt extrusion, Montmorency, France; or FRED; Microvention, Tustin, CA) were used (Fig. 2 and 3).
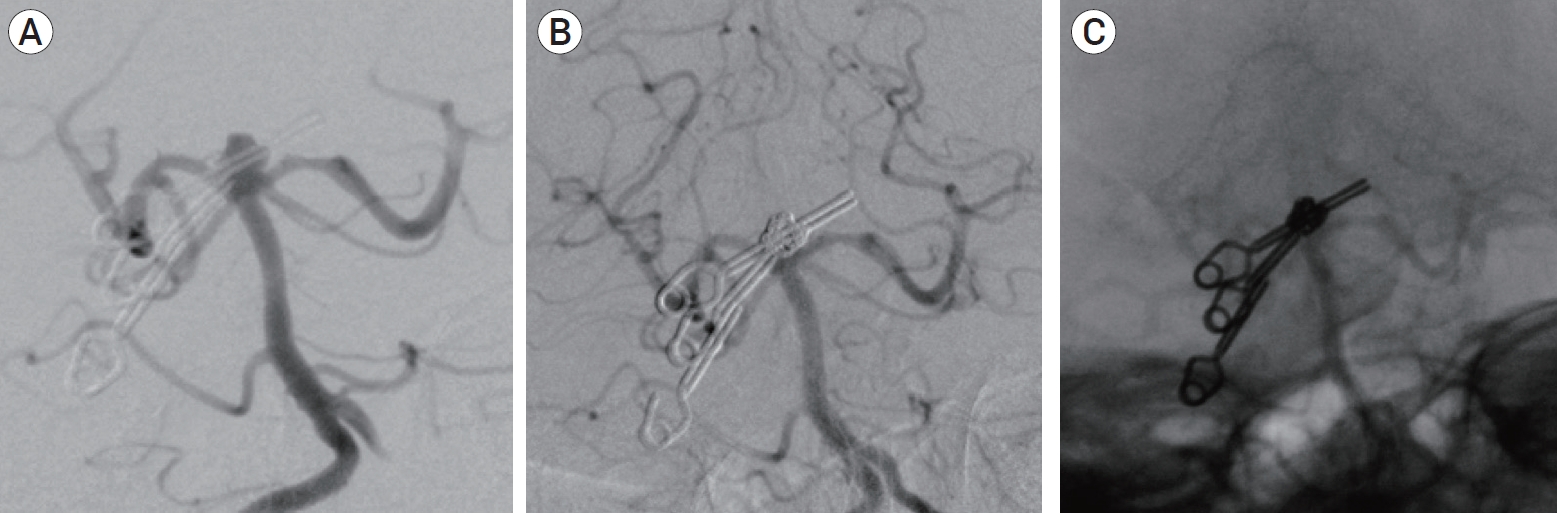
(A) Control angiography after clipping revealing a residual basilar tip aneurysm. (B) Control angiography after simple-coiling embolization demonstrating complete aneurysm occlusion. (C) Note the cast of coils inside the aneurysm and the two clips.
(A) Angiography showing a residual ophthalmic segment aneurysm after surgery. (B, C) 3D rotational angiography demonstrating the clip adjacent to the aneurysm neck. (D) 6-month control angiography revealing complete occlusion of the aneurysm after treatment with a flow diverter device. (E, F) 3D angiography demonstrating complete shrinkage of the aneurysm.
The patients were awakened from general anesthesia and then admitted to the intensive care unit. Patients harboring unruptured aneurysms were usually discharged 48 hours after treatment. Patients with ruptured aneurysms were discharged several days later depending on the neurological status.
Data collection
All data were collected prospectively. The preoperative data included age, sex, clinical presentation, interval between surgery and EVT, modified Rankin Scale (mRS) score, aneurysm location, and aneurysm size (dome, neck, and dome/neck ratio).
The operative data included treated aneurysm, presence of angiographic vasospasm in ruptured aneurysms, type of EVT, technical complications, and immediate anatomic result (Montreal scale). Immediate occlusion was not considered for aneurysms treated with a flow diverter.
The postoperative evaluation included neurological complications, mRS scores, occlusion rates, mortality and morbidity rates, recanalization, and retreatment rates.
Radiological follow-up for the study period included 3 digital subtraction angiography sessions: immediately after the procedure, after 6 months and after 1 year. A digital subtraction angiography (DSA) was again performed at 3-years post-treatment. For aneurysms treated with flow diverters, if the aneurysm was totally occluded at 1-year control, a long term DSA was not performed. Based on the clinician’s suspicion for recurrence or the presence of significant risk factors for regrowth or recurrence, additional follow-up was performed with magnetic resonance imaging. Images were analyzed by an independent neuroradiologist. Clinical follow-up included an evaluation before the procedure and then 30 days, 6 months, and 1 year after the procedure.
Statistical analyses
Categorical variables were presented as absolute numbers and percentages, and continuous variables were presented as the means±standard deviation (SD)s and ranges.
Adjusted marginal logistic regression (univariable and multivariable) was performed to verify the effect of variables of interest on total occlusion and recanalization rates at 1 year. The stepwise method was used to select the variables. Following the Forward method, a univariable analysis was performed. Fisher’s exact and Chi-square tests were used to verify the association between categorical variables. For numerical variables, the Mann-Whitney test was used. The variables with a p value less than 0.25 were selected for the multivariable analyses.
For the selected variables, the Backward method, in which the least significant variable is repeatedly eliminated until only significant variables remain, was performed through marginal logistic regression and logistic regression. A level of significance of 5% was accepted. The Hosmer-Lemeshow test and pseudo R2 statistics were used to verify the goodness of fit of the model.
Two measured outcomes, namely, complications and morbidity after 1 year, did not fit the model. They were presented as a descriptive analysis. The morbidity was analyzed in comparison to other variables.
R version 3.6.1 software (Institutes for Statistics and Mathematics, Vienna, Austria) was used.
RESULTS
Between May 2010 and September 2018, 70 patients harboring incompletely occluded intracranial aneurysms after clipping met the inclusion criteria in 5 centers. Endovascular treatment was successfully completed in all patients.
Patient demographics
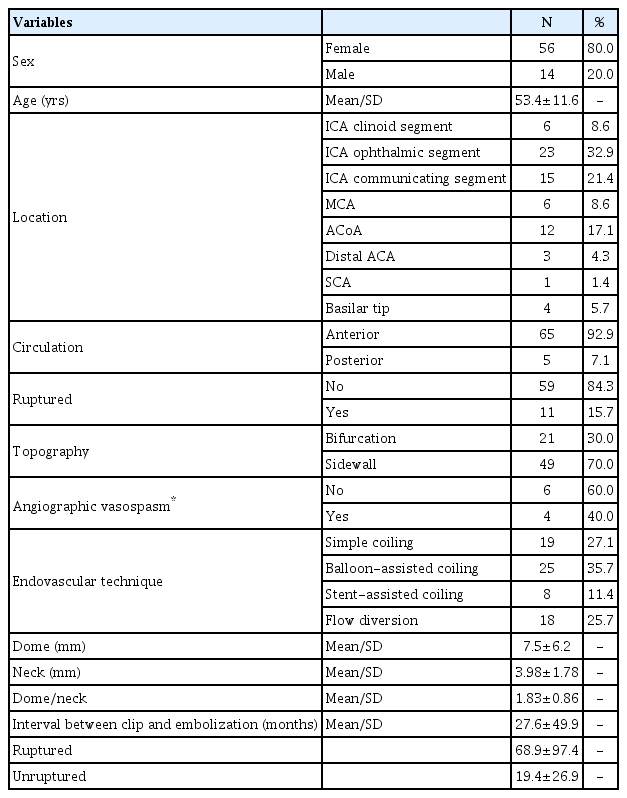
Among 70 patients, 56 were women (80%), and their mean age was 53.4 years old (range: 24-77 years old) (Table 1). Most patients (84.3%) had unruptured aneurysms. The mRS score before treatment was 0 in 49 patients, 1 in 11 patients, 2 in 7 patients, and >2 in 3 patients.
Aneurysm characteristics
The mean aneurysm size was 7.5 mm in diameter (range: 3-40 mm). The most frequent location was at the ophthalmic segment of the internal carotid artery (32.9%). Most aneurysms were located at a sidewall (70%) in the anterior circulation (92.9%).
Treatment characteristics
The mean interval between surgical treatment and embolization was 27.6 months±46.9 (range, 1 day to 23 years). The most frequently used technique was BAC (35.7%) (Table 1). Considering the aneurysms treated until day 21 after subarachnoid hemorrhage, 40% had associated vasospasm on the day of the treatment.
Division of the sample into four groups according to the treatment technique showed that baseline patient and aneurysm characteristics were similar between groups, except in the SAC group, which demonstrated a higher proportion of bifurcation aneurysms, and in the FD group, which exhibited a higher proportion of sidewall aneurysms (Table 2).
Treatment outcomes
The total occlusion rate was 73.1% on immediate post-procedure angiography (excluding aneurysms treated with flow diverter). Treatment outcomes after 1 year comprised 69 aneurysms because 1 patient died. Total aneurysm occlusion, assessed by 1-year angiography, was observed in 75.3% of the aneurysms. Neck remnants were present in 20.3% and residual filling of the sac in 4.4%. Recanalization after endovascular retreatment was observed in 14.5% of the cases (Table 3). In 3 aneurysms, because of the size of the recanalization (diameter >3 mm), as evaluated with 1-year angiography, retreatment was planned.
Intraoperative complications were observed in 6 (8.6%) patients, 5 of them with unruptured aneurysms. Three patients presented with thromboembolic complications, including a patient with a ruptured anterior communicating aneurysm. These patients were treated with intra-arterial administration of abciximab and had uneventful recoveries.
One case of aneurysm rupture during coiling was observed. Fast implantation of additional coils stopped the bleeding. Vessel perforation occurred in a patient during an exchange maneuver for flow diverter deployment. The bleeding was solved with the inflation of a balloon. One case of retroperitoneal hemorrhage was treated conservatively.
There was no morbidity or mortality due to procedure-related complications.
During the first year after treatment, 1 (1.4%) patient harboring a ruptured anterior communicating artery aneurysm died due to complications of vasospasm. Permanent morbidity occurred in 2 (2.9%) patients due to complications related to subarachnoid hemorrhage (both with ruptured posterior communicating segment aneurysms subjected to balloon remodeling treatment). Another 2 patients with ruptured aneurysms presented with transient morbidity not related to the treatment, but they fully recovered.
Statistical analyses
Total aneurysm occlusion after 1 year
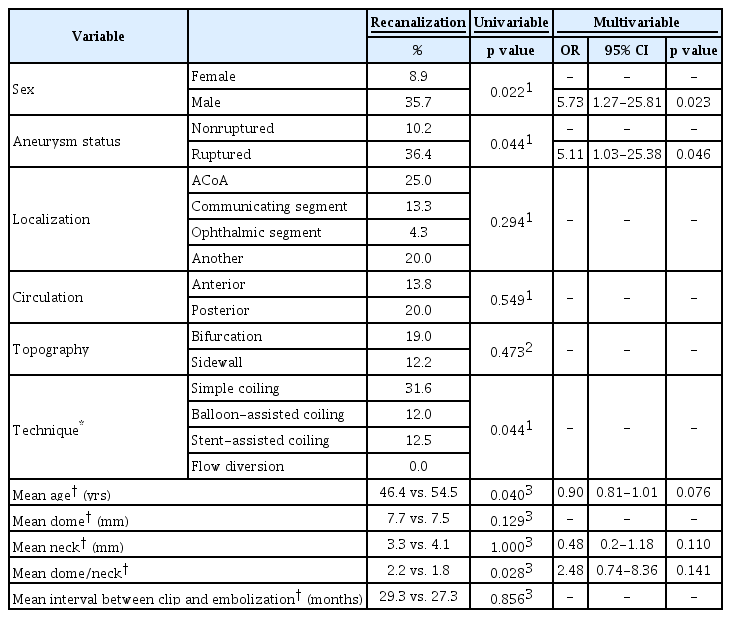
Univariable analysis demonstrated that there was a positive influence of unruptured status of the aneurysm and a reduced dome/neck ratio on total occlusion. Additionally, treatment with a flow diverter device was associated with higher rates of occlusion, as all aneurysms treated with this technique were completely occluded after 1 year (Table 4).
Multivariable analysis revealed that ruptured aneurysms were less prone to be totally occluded after 1 year of treatment (odds ratio [OR], 0.08, 95% confidence interval [CI], 0.02-0.34). The dome/neck ratio was not significant in this analysis. The effect of flow diversion treatment could not be tested because this variable did not fit the model.
Recanalization rates
Univariable analysis demonstrated that recanalization of the aneurysm after EVT was more frequent in male patients, ruptured aneurysms, aneurysms treated with a technique other than flow diversion, younger patients, and aneurysms with a higher dome/neck ratio (Table 5).
Multivariable analysis confirmed that male sex (OR, 5.73; 95% CI, 1.27-25.81) and ruptured status (OR, 5.11; 95% CI, 1.03-25.38) were associated to higher rates of recanalization. Again, the variable of the treatment technique did not fit the model.
Morbidity and mortality
Univariable analysis showed that ruptured aneurysms and advanced patient age were associated with the occurrence of death and permanent morbidity. Neither variables were suitable for the multivariable model. The morphological characteristics of the aneurysms, treatment techniques, localization, and intervals between surgical treatment and embolization did not predict a worse clinical outcome.
DISCUSSION
Despite the high degree of technical expertise gained by those who have dedicated their careers to cerebrovascular surgery, good results are not uniformly achieved, and residual or recurrent intracranial aneurysms can occur after surgical treatment [7,9,10,29].
The causes of failure include a “slipped clip”, when clips with relatively low closing forces slides out of the neck of the aneurysms; improper placement of the clip; and regrowth of a totally occluded aneurysm [5,7,29].
The natural history of aneurysm remnants after surgical clipping is marginally known. Therefore, the prognostic significance of these rests remains controversial. Many residua are believed to undergo spontaneous thrombosis, although a small number have been noted to enlarge and even bleed [2,25]. The risk of hemorrhage from an aneurysm rest may be approximately 0.8% to 1.9% per year [2,6]. A case series evaluating only residual aneurysms after EVT revealed that previously ruptured aneurysms were at a higher risk of hemorrhage than aneurysms that had never bled [18]. Although small, the danger of bleeding persists for years and may result in serious and even fatal consequences [2].
Considering the relative low risk of aneurysm remnant rupture, retreatment-related complications must be considered. Patient age and the degree of occlusion are the most significant predictors of remnant growth and rupture [10,29]. Therefore, depending on the size of the remnant, the aneurysm must be retreated. It is usually advocated, especially in young people.
Reoperation has been traditionally indicated for patients with incompletely occluded aneurysms after clipping. But this procedure can be associated with higher risks of complications than the initial operation. Not infrequently, anatomic difficulties encountered during the original surgery account for the persistence of an aneurysm remnant [25]. Scar adhesions can obscure the anatomy and tether the aneurysm, making reoperation more technically demanding [7,10,24]. The previous clip can be in a compromising position, obstructing the dissection, which makes reclipping challenging. Removal of an old clip can be dangerous [5,9]. Rates of 7% for major morbidity and 5.2% for mortality were reported [5]. Giannotta et al. observed that 25% of reoperative procedures were complicated by premature rupture [7].
The embolization of previously clipped aneurysms has emerged as an alternative method to avoid the difficulties of reoperation [8,14,15,19,25]. Rabinstein et al. published the first large case series in 2002, that included 21 ruptured and unruptured aneurysms. Total occlusion was achieved in 81% of the cases using simple coiling or balloon-assisted coiling. No major complications were associated with the endovascular procedure [25].
The largest case series to date included 60 residual or recurrent clipped aneurysms [19]. Treatment was performed by SC in 42%, SAC in 25%, BAC in 13%, FD in 13%, stenting alone in 5%, and flow diversion with coiling in 2%. Excluding patients treated with a flow diverter or stenting alone, complete initial occlusion was achieved in 55%. On the other hand, at the last follow-up, complete aneurysm occlusion was seen in 79% of cases. The permanent neurological morbidity rate was 3%, and the mortality rate was 2%.
Other case series have revealed that the immediate complete aneurysm occlusion rate varies from 53% to 86%, and the procedure-related morbidity and mortality varies from 1% to 5.3% [14,15,19].
The current series presented similar results. Total aneurysm occlusion after 1 year was observed in 74.3% of the aneurysms. A composite of total occlusion/residual neck remnants resulted in a rate of 95.7%. Permanent morbidity and mortality occurred in 4.3% of cases, none of which were related to the procedure. Therefore, the risk of treatment-related complications (even when adjunctive techniques are required, such as BAC, SAC, and FD) seems to be at least comparable to, if not smaller than, the risk of the conservative management.
Previous studies addressing the whole population of embolized aneurysms seem to have lower total occlusion rates and higher recurrence rates than the current series. Pierot et al. reported total aneurysm occlusion in 63%, neck remnants in 22.5% and aneurysm remnants in 14.6% [21]. A large study revealed recurrence in 28.6% of the aneurysms at a mean follow-up of 12 months; 5.5% were amenable to retreatment [23]. Direct comparison should be performed with criticism once aneurysm samples are different, and these studies were published before the era of flow diversion.
We observed that retreatment with flow diversion predicted complete aneurysm occlusion. This is in accordance with other case series that included residual aneurysms after clipping and coiling, which exhibited a rate of total occlusion of 93.3% to 100% 1 year after flow diversion [4,17]. Flow diversion in a pooled analysis of 3 large studies, including not only failed clipped aneurysms, demonstrated a 1-year complete occlusion rate of 85.5% [12].
Additionally, ruptured aneurysms were associated with higher rates of incomplete occlusion, recurrence and poor outcomes after 1 year. Advanced age was also associated with worse outcomes. Previous studies reported that rupture status was the most important predictor of disability and death [14,25].
Two different scenarios are faced during retreatment. In some aneurysms, incomplete clip occlusion results in a narrow neck. On the other hand, in other cases, partial occlusion of the aneurysm dome creates an aneurysm with a very unfavorable dome/neck ratio for EVT. For unruptured aneurysms, the flow diverter can be successfully used to overcome this anatomical challenger. Moreover, in some situations, even with the use of 3D angiography, the use of multiples clips impairs the visualization of the aneurysm neck. Unfortunately, these anatomical changes after clipping were not systematically measured in this study.
The major strength of this study is that it is the largest case series regarding embolization of incomplete clipped aneurysms. No eligible patients were lost to follow-up. Predictors of occlusion, recanalization and clinical outcome could be identified. On the other hand, no randomization was performed, especially regarding the treatment technique. Thus, this limits the ability to identify outcome differences between the techniques. Additionally, in most cases immediate post-clipping angiography was not available. Therefore, a difference between residual or recurrent aneurysm could not be accessed. Finally, most aneurysms were located at a sidewall, with a low representation of middle cerebral artery bifurcation aneurysms. Consequently, the findings of this study should be considered preliminary.
CONCLUSIONS
Endovascular treatment of recurrent or residual aneurysms after surgical clipping was safe and efficacious. Flow diversion seems to be associated with better anatomical results. A more rigid study, a larger group of patients, and long-term follow-up are required to provide stronger conclusions about the best approach for residual clipped aneurysms.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.