Utility of skull X-rays in identifying recurrence of coiled cerebral aneurysms
Article information
Abstract
Objective
A high rate of cerebral aneurysm recurrence following endovascular coiling has prompted the use of digital subtraction angiography (DSA) for interval follow-up. However, the utility of skull x-rays as an alternative screening method for aneurysm recurrence is unproperly characterized.
Methods
Retrospective review of a prospective registry of ruptured and unruptured cerebral aneurysms. Anteroposterior and lateral skull x-rays were obtained immediately at the end of the procedure and at 6-month follow-up. Aneurysm recurrence was defined by comparing post-procedure and 6-month DSA imaging. A true positive was defined as a change in coil mass morphology on at least one projection with aneurysm recurrence on DSA, and a true negative defined as a stable coil mass on both projections and no recurrence on DSA. Receiver operating characteristic area under the curve (AUC) statistics was used to assess the performance of skull x-rays in identifying aneurysm recurrence.
Results
A total of 118 cerebral aneurysms were evaluated with DSA imaging and skull x-rays. A change in coil mass morphology on one projection of skull x-rays correctly detected all true recurrences with a sensitivity of 100% (95% confidence interval [CI], 91-100%). Skull x-rays failed to identify a stable aneurysm coil mass in 15 cases, with a specificity of 79% (68-88%). Skull x-rays performed with AUC 0.8958 (95% CI, 0.8490-0.9431) in identifying aneurysm recurrence.
Conclusions
The findings of our study suggest that skull x-rays may represent a low-cost, non-invasive screening tool to rule out aneurysm recurrence, which can potentially aid in decreasing the utilization of DSA in the follow-up of patients with coiled cerebral aneurysms.
INTRODUCTION
Use of endovascular coiling (EC) for treatment of cerebral aneurysms continues to be widely adopted, with randomized clinical trials demonstrating a lower rate of poor outcomes compared to surgical clipping (SC) [17-19]. However, coiled aneurysms recur more often than those treated with SC, with recurrence rates ranging between 20% to 36% and retreatment rates from 10% to 17% [5-7,9,10,13,20,26]. Owing to its high recurrence rate, digital subtraction angiography (DSA) and magnetic resonance angiography (MRA) have been utilized by neurointerventionists as part of the imaging protocol during interval follow-up. However, the associated cost and risks of this imaging protocols may limit their use.
Although DSA is the gold standard to assess coil stability and vessel patency following EC, it carries a 0.5-3% risk of thromboembolic events, access-related complications, contrast nephropathy, and radiation exposure [2,8,23]. Moreover, DSA is also time consuming due to its longer acquisition time, as most patients have to spend around 6-8 hours in the hospital for the study, which may contribute to a decreased compliance to follow-up. MRA has emerged as an alternative imaging study for evaluating aneurysm recurrence instead of DSA due to its cost-effectiveness [12,21]. However, coils may interfere with its accuracy and sensitivity, and some patients may not be candidates to undergo MRA imaging (e.g., claustrophobia not amenable to sedation, pacemakers, metallic foreign bodies), which may limit its use. MRA acquisitions techniques such as silent MRA and 3T MRA may overcome coil interference, although its interpretability has not been thoroughly described [14,21,24,25]. Furthermore, both DSA and MRA are associated with significant costs to patients. Based on 2018 Centers for Medicare & Medicaid Services, the total cost fee schedule for a single internal carotid artery angiogram is $4,501 and $1,078 for an MRA, with these costs accumulating to a significantly expensive health care bill with each follow-up.
In this study, we sought to evaluate the performance of skull x-rays to detect aneurysm recurrence in patients with ruptured and unruptured aneurysms who underwent EC for aneurysm occlusion. We hypothesize that use of skull x-rays may represent a sensitive test to rule out aneurysm recurrence, and identify patients who may benefit from undergoing DSA, thus minimizing costs and risks to patients.
MATERIALS AND METHODS
Study design
This study was reviewed and approved by the Institutional Review Board. We conducted a retrospective review of a prospective registry of ruptured and unruptured cerebral aneurysms occluded with EC and evaluated by AP and lateral skull x-rays obtained at the end of the coiling procedure (i.e., baseline imaging). At 6-month follow-up, all aneurysms were evaluated by DSA imaging. Coiled aneurysms with an incomplete imaging protocol or with inadequate image acquisition were not included in the analysis.
Imaging acquisition and labeling
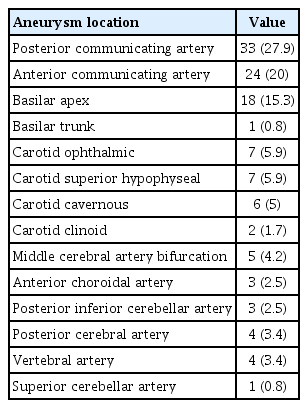
To maintain the consistency of the acquisition of skull x-rays at baseline and 6-month follow-up, bony landmarks served as reference to ensure correct patient positioning of the acquisition of straight AP and lateral projections. Examples of skull x-ray acquisition are shown in Fig. 1. Comparison of baseline (i.e., immediate post-procedure) and 6-month skull x-rays served to identify changes of the coil mass, defined as compaction, unwinding, or alteration in its shape or configuration. Example of these coil mass changes are shown in Fig. 2.

Acquisition of skull x-rays at the end of the coil embolization procedure (A, C) and at 6-month follow-up (B, D). Anteroposterior projection (A, B) was obtained with the patient looking straight to the front. A lateral projection (C, D) was obtained with alignment of the external auditory canals.
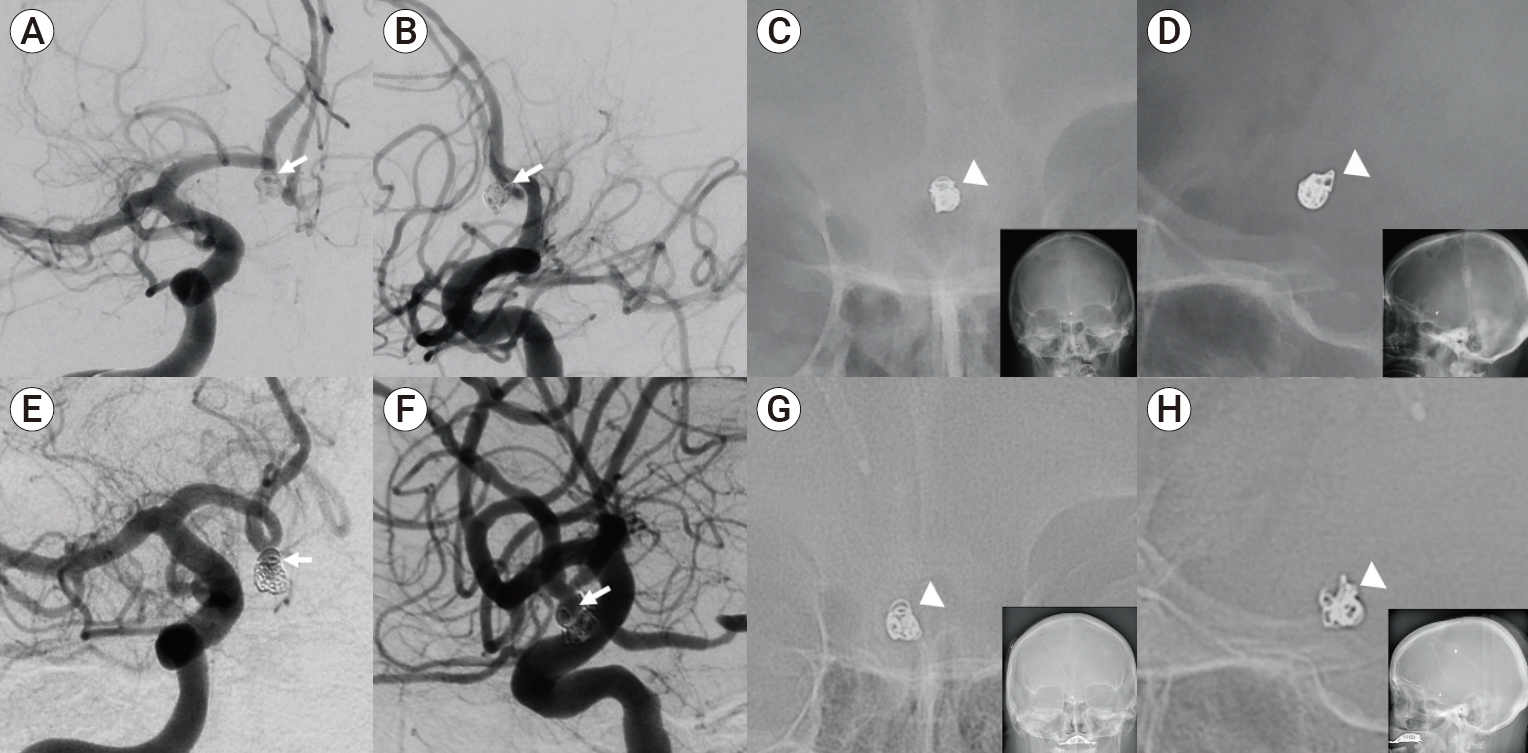
Illustrative case of aneurysm recurrence. Patient presented with an A1-A2 junction cerebral aneurysm treated with balloon assisted coil embolization as shown in the oblique AP and lateral views (1A and 1B, white arrow) on DSA. At the end of the coiling embolization procedure, AP and lateral projections of skull x-rays were taken showing a coil mass (C and D, white arrowhead). At 6-month follow-up, a set of skull x-rays and DSA were performed. Oblique AP and lateral views on DSA showed aneurysm recurrence (E and F, white arrow). On skull x-ray AP and lateral views (G and H, white arrowhead), there is changed shape (unwinding) of the coil mass, corresponding with aneurysm recurrence identified on DSA. DSA, digital subtraction angiography.
DSA performed at 6 months was used to confirm aneurysm stability based on findings of aneurysm recurrence. Aneurysm recurrence was defined by the modified Raymond-Roy classification, as previously described in the literature [16]. A true positive result was defined as evidence of a change in coil mass in at least one of the AP and lateral projections that corresponded with aneurysm recurrence on follow-up DSA and a false positive result as a change in coil mass on skull x-rays with evidence of coil mass stability and no aneurysm recurrence on DSA. A true negative result was defined as the absence of coil mass change that corresponded with absence of aneurysm recurrence on DSA, and a false negative result as no change in coil mass on skull x-rays with evidence of aneurysm recurrence on DSA.
Two independent, experienced neurointerventionists reviewed and labeled the skull x-ray and DSA studies. The readers were blinded to the patient identity, treatment outcome, DSA findings, and the interpretation of the other reader. Any discrepancies between readers were resolved by a consensus, which was finally used for analysis.
Statistical analysis
Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated with 95% confidence intervals using the Exact method. Receiver operating characteristic area under the curve (AUC) statistics were used to assess the performance of skull x-rays in identifying aneurysm recurrence. Statistical analyses were performed with Stata IC (Release 14.0, StataCorp, College Station, TX, USA).
RESULTS
A total of 118 cerebral aneurysms were reviewed. 76 aneurysms (64%) were associated with subarachnoid hemorrhage (SAH), whereas 42 aneurysms (36%) were unruptured. At 6-month follow-up, 61 aneurysms (43%) showed changes on coil mass while only 47 (39%) showed true aneurysm recurrence on DSA. Among those showing recurrence on DSA, 16% required reintervention, with 14% and 17% for unruptured and ruptured aneurysms, respectively. Location of the cerebral aneurysm in the cohort is detailed in Table 1.
Patient cohort
The imaging protocol consisting of skull x-rays and DSA for screening of aneurysm recurrence was utilized in 108 patients. Among patients in the cohort, median age was 55.5 years (range, 16-81 years), 65% were female, 51% had hypertension, 32% were active smokers, 13% had diabetes mellitus and 6% had a history of coronary artery disease. 34 patients (31%) had an unruptured aneurysms, while 74 patients (69%) presented with SAH. Among those presenting with SAH, 23% presented with a Hunt-Hess 4-5 on arrival to our institution. Average follow-up time was 13.26 months (range, 6-46 months), for a total of 1188 patient-months.
Skull x-rays performance
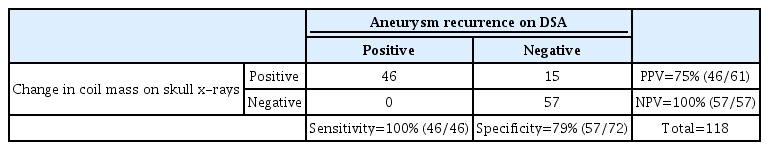
Skull x-rays performed with an AUC of 0.8958 (0.8490-0.9431) in identifying aneurysm recurrence. As shown in Table 2, skull x-rays ruled out aneurysm recurrence with a sensitivity of 100% (95% confidence interval [CI], 92-100%) and a NPV of 100% (95% CI, 94-100%). Among 57 aneurysms that showed no aneurysm recurrence on DSA, all 57 aneurysms showed no change in the coil mass on the skull x-rays. Skull x-rays correctly ruled out aneurysm recurrence in previously ruptured and unruptured aneurysms, with a sensitivity of 100% and a NPV of 100% for both (Table 3, 4).
Diagnostic performance of skull x-rays in detecting aneurysm recurrence at 6-month follow-up of all coiled aneurysms (N=118)

Diagnostic performance of skull x-rays in detecting aneurysm recurrence at 6-month follow-up of unruptured aneurysms treated with a coiling procedure

Diagnostic performance of skull x-rays in detecting aneurysm recurrence at 6-month follow-up of ruptured (B) aneurysms treated with a coiling procedure
In identifying aneurysm recurrence, skull x-rays performed with a specificity of 79% (CI 68-88%) and a PPV of 75% (95% CI 63-86%) (Table 2). Changes in coil mass on skull x-rays were identified in 61 aneurysms, with 33 (54%) aneurysms showing changes in both projections and 28 (46%) in only one projection. Among those 61 aneurysms, 46 aneurysms had evidence of aneurysm recurrence on DSA with the remaining having no evidence of recurrence on DSA. An illustrative case of a false positive result is shown in Fig. 3.

Illustrative case of a false positive in a ruptured anterior communicating aneurysm. AP (A1) and lateral (A2) DSA projections showing the aneurysm prior to coil embolization. AP (B1) and lateral (B2) DSA projections and AP (B3) and lateral (B4) projections of skull x-ray obtained immediately after coiling. At 6-month follow-up, AP (C3) and lateral (C4) projections of skull x-ray both demonstrated the changed shape of the coil mass, but no evidence of recurrence or coil compaction was observed on angiography (C1 and C2). At the 2-year follow-up, AP (D3) and lateral (D4) skull x-ray projections showed changed coil shape on the lateral view, but this was not confirmed by angiography (D1 and D2). DSA, digital subtraction angiography.
DISCUSSION
In this study evaluating the performance of skull x-rays in ruling out aneurysm recurrence following EC at 6-month follow-up, the absence of changes in coil mass on skull x-rays correctly identified a stable coiled aneurysm (Sensitivity 100% [95% CI 92-100%]; NPV of 100% [CI 94-100%]). Skull x-rays showed a good performance in identifying aneurysm recurrence (Specificity of 79% [CI 68-88%]; PPV of 75% [95% CI 63-86%]), with an accuracy of 89% (AUC 0.8958 [0.8490-0.9431]) [1,5-7,9,10,13-15,20,26].
Imaging protocol for identifying aneurysm recurrence
A high rate of aneurysm recurrence following EC demands close vigilance to assess the need for reintervention to prevent bleeding [5-7,9,10,13,20,26]. In our cohort, we identified a 39% recurrence rate within 6 months following EC. Given the risk of recurrence, first follow-up is scheduled within 6 months after the procedure, followed by a yearly follow-up, with most practitioners utilizing DSA to identify aneurysm recurrence [1,15]. Although MRA has been also used [14], DSA may still be required to confirm the presence of aneurysm recurrence and assess vessel patency for aneurysms occluded with a stent-coiling approach.
In our study, our imaging protocol was standardized and comparisons were performed using consistent skull x-ray projections at the end of the procedure and at 6-month follow-up, thus reducing the chance of a type II error associated with inter-subject variability. As a result, we found that skull x-rays have a sensitivity of 100% in detecting aneurysm recurrence in unruptured and previously ruptured aneurysms. However, modified Raymond-Roy Class II (i.e., residual neck) aneurysms could potentially be missed on skull x-rays projections, as changes in the coil mass may not be evident in this scenario. However, in our study all aneurysms showing a modified Raymond-Roy Class II recurrence on DSA were correctly identified on skull x-rays.
The goal of a screening test is to rule out patients without the disease (e.g., aneurysm recurrence) to reduce the need of further unnecessary diagnostic workup. Given the results of our study, a simpler follow-up algorithm for patients with aneurysms treated with EC, either ruptured or unruptured, could be performed. AP and lateral skull x-rays at the end of EC procedure and at 6-month follow-up may be compared, and if a change in coil mass is noted, then the standard catheter angiogram can be recommended, but if not, DSA can be postponed 6 months later, as aneurysm recurrence is most common within the first year, although some may recur within 1-3 years following treatment [1,18,22].
Skull x-rays screening for aneurysm recurrence
Previous studies have reported the potential use of skull x-rays as an alternative in the assessment of aneurysm recurrence. Hwang et al. [11] retrospectively reviewed 100 DSA studies and skull x-rays at follow-up of 82 aneurysms in 78 patients who underwent EC for aneurysm repair. 97% of the findings on skull x-rays correlated with angiographic findings; however, DSA imaging was performed inconsistently during follow-up, ranging between 1-54 months after the embolization procedure. In a study by Connor et al. [3], 43 sets of skull x-rays in AP and lateral projections showed a sensitivity of 93% and specificity of 71% in identifying aneurysm recurrence in 38 patients with coiled aneurysms. However, there was inconsistency in the projections of skull x-rays and DSA studies that may have resulted in their lower sensitivity and specificity. Cottier et al. [4] compared the use of a single skull x-ray projection in detecting recurrence of coiled aneurysms and reported a sensitivity of 78%. The low sensitivity of skull x-rays in the study can be attributed the use of a single projection representing the best aneurysm visualization during follow-up, as it is difficult to assess a 3-dimensional object with a single projection. Compared to these studies, in our study we consistently acquired skull x-rays projections, performed standardized follow-up imaging at 6 months and evaluated 2 projections to obtain a better representation of the coil mass.
Limitations
Our study has several limitations, including a single-center experience with a small sample. Second, the assessment of changes in coil mass may be subjective and prone to inter-reader variability. However, use of bone landmarks allows to ensure for consistency and interpretability. Third, aneurysms with a higher risk of regrowth of the aneurysm neck, such as small aneurysms (i.e., <7 mm) in the paraclinoid region, may not be easily identified on skull x-rays as the bone structures may reduce the visibility of the coiled aneurysm. Finally, aneurysms treated with a stent-coil approach may still require DSA at follow-up to assess the patency of the parent vessel.
CONCLUSIONS
The findings of our study suggest that use of skull x-rays for screening of aneurysm recurrence offer a safe, non-invasive and sensitive alternative that may guide neurointerventionists at 6-month follow-up. Thus, skull x-rays may help selecting patients who can benefit from DSA imaging to identify aneurysm recurrence. Implementation of this screening protocol may result in lower costs in patient care, decreased risk of thromboembolic events, and higher patient compliance during interval follow-up.
Notes
Disclosure
Peng Roc Chen is a recipient of NIH grant and Stryker’s Research Grant.