Role of microsurgery for treatment of posterior circulation aneurysms in the endovascular era
Article information
Abstract
Objective
Several studies have reported that the outcomes of endovascular treatment were superior to those of microsurgical treatment for posterior circulation aneurysms. Thus, this study compared outcomes of endovascular and microsurgical treatment for posterior circulation aneurysms and assess the usefulness of microsurgery in these patients.
Methods
Outcomes were retrospectively evaluated after endovascular and microsurgical treatment of 621 posterior circulation aneurysms. The 621 aneurysms included 187 treated by surgical clipping and 434 treated by endovascular coiling.
Results
In patients with unruptured aneurysms the rates of residual lesions and retreatment were significantly lower in those who underwent microsurgical than endovascular treatment. However immediate postoperative and 6 month follow-up Glasgow outcome scale (GOS) scores did not differ significantly in the two groups. In patients with ruptured aneurysms, the rates of residual lesions and retreatment were significantly lower in the microsurgery than in the endovascular treatment group. Even so immediate postoperative and 6 month follow-up GOS scores did not differ significantly in the two groups.
Conclusions
Endovascular treatment has increasingly become an alternative modality for microsurgery in posterior circulation aneurysm, whereas the indication for microsurgery is greatly reduced. However, the absolute number of microsurgery is maintained showing that it is a still valuable technique, as advances in endovascular or stent-assisted coiling have not solved many of the challenges inherent in the management of complex aneurysms. Hence, the advantages and limitations of both modalities must be carefully concerned in posterior circulation aneurysm to obtain favorable outcome.
INTRODUCTION
Posterior circulation aneurysms account for approximately 8–15% of all cerebral aneurysms.11)21)34)35) The rupture risk of posterior circulation aneurysms is about 3–5% per year, higher than that of anterior circulation aneurysms.5)30)38)41)42) Treatment of posterior circulation aneurysms is difficult because they are located in a deep position and are surrounded by important structures, including the cranial nerves, brainstem, and several arteries that perforate the brain stem. Moreover, treatment of posterior circulation aneurysms is associated with a relatively high complication rate.
Since the first clipping in 1954, microsurgery has been the treatment of choice for posterior circulation aneurysms.12) However, the development of endovascular techniques has resulted in higher numbers of aneurysms, especially posterior circulation aneurysms, being treated by endovascular methods.2)26)39) Endovascular methods have yielded slightly better results than microsurgery in the treatment of posterior circulation aneurysms.11)21)23) However, the durability of results obtained with the endovascular technique remains unclear, as some patients require surgical treatment due to technical failure or repeated coil compaction.16)32) The goals of aneurysm therapy include obliteration of the aneurysm, prevention of recurrence after treatment, preservation of blood flow through adjacent vessels, and preservation of surrounding neurological structures and the patient’s neurological condition, whether the aneurysm is ruptured or unruptured. The present study retrospectively reviewed the 15 years experience of our center in treating posterior circulation aneurysms. The clinical and radiological characteristics and treatment outcomes of patients with ruptured and unruptured aneurysms treated microsurgically and endovascularly were compared. These findings provide insight into the role and necessity of microsurgery in the treatment of posterior circulation aneurysms.
MATERIALS AND METHODS
This study was approved by the institutional review board of our institution prior to data collection.
Inclusion criteria
From 2000 to 2015, 7,594 cerebral aneurysms were treated microsurgically or endovascularly at our hospital. From this cohort, 684 (9.0%) posterior circulation aneurysms were diagnosed from 616 patients. The medical, surgical, and radiologic records of patients with the posterior circulation aneurysms were reviewed and analyzed. Patients followed-up for less than 6 months were excluded, although patients who died of intracranial hemorrhage associated with rupture of the aneurysm or complications related to treatment of the aneurysm were included, regardless of the duration of follow-up. 684 posterior circulation aneurysms were enrolled of whom 621 aneurysms were available after inclusion and exclusion criteria. Prior to treatment, all patients underwent computerized tomography (CT) and magnetic resonance (MR) imaging to confirm intracranial hemorrhage, including subarachnoid hemorrhage. If a ruptured aneurysm was strongly suspected but intracranial hemorrhage was not clear on CT, cerebrospinal fluid (CSF) obtained through lumbar puncture was analyzed to confirm subarachnoid hemorrhage. Before neurosurgical treatment, all patients underwent conventional cerebral angiography to determine aneurysm size, location, and treatment plan.
Based on conventional cerebral angiography imaging, posterior circulation aneurysms were subdivided anatomically into aneurysms of the basilar apex, posterior cerebral artery (PCA), superior cerebellar artery (SCA), anterior inferior cerebellar artery (AICA), basilar trunk, vertebrobasilar (VB) junction, vertebral artery (VA), posterior inferior cerebellar artery (PICA).
Aneurysm size was determined by measuring its longest diameter on three dimensional reconstruction of conventional cerebral angiography, or, if conventional angiography was not possible, on cerebral CT angiography. The aneurysms were classified by size as small (<10 mm), large (10–25 mm), and giant (>25 mm). The diagnosis of dissecting aneurysm was evaluated from the radiologic findings like 1) visualization of double lumens in MR or 2) pearl and string sign (ectatic segment with stenosis), 3) contrast stagnation and 4) blood like blister.27)
General indication
In our institute, the treatment decisions of cerebral aneurysm including treatment modality and techniques, were at the discretion of the attending neurosurgeons or neurointerventionist. The decision of treatment modality has been greatly changed after the introduction of endovascular treatment. Therefore, the therapeutic paradigm has changed in response to advances in its popular endovascular technique.
In the early 2000s, microsurgery was performed when the aspect ratio of aneurysm was less than 1:2, or complex cases like giant or fusiform aneurysm where bypass technique was mandatory. Usually endovascular treatment was recommended in other cases.
From the 2010s, endovascular treatment was mostly considered for ruptured posterior circulation aneurysm case. Microsurgery was considered in following situations: 1) broad neck aneurysm with aspect ratio less than 1:2; 2) multiple aneurysms including anterior circulation aneurysm; 3) whenever bypass was needed, such as fusiform aneurysm cases; or 4) clinically relevant arterial branch incorporated into the aneurysm neck, body or dome.
Microsurgery (Fig. 1)

(A) Three dimensional reconstruction of conventional cerebral angiography showing a fusiform aneurysm at the PCA. (B) A Coronal image with a T2 sequence at the level of the PCA dissecting aneurysm obtained through high-resolution magnetic resonance imaging. Black arrow indicates aneurysmal dilatation. (C) A Coronal image with a gadolinium-enhanced T1-weighted sequence at the level of the PCA dissecting aneurysm obtained through high-resolution magnetic resonance imaging. White arrow indicates intimal flap and double lumens. (D) Conventional cerebral angiography showing trapping of right proximal P2 segment of PCA. (E) Conventional cerebral angiography showing patent anastomosis of STA to the right distal P2 segment. PCA, posterior cerebral artery; STA, superficial temporal artery.
Various surgical approaches have been applied to the treatment of posterior circulation aneurysms, depending on their location (Table 1).8) The surgical approach was determined by assessing the location and size of the aneurysm, the parent artery, the cranial nerve, and the bony structure, as well as their relationships (Table 2). Bypass surgery (e.g., superior temporal artery [STA]-PCA, STA-SCA, occipital artery [OA]-PICA) was planned when parent artery flow decreased during the process of aneurysm obliteration or when parent artery sacrifice was expected. If bypass surgery was expected, the STA or OA was dissected first.
If necessary, especially in patients with a giant aneurysm, clipping to reduce the internal pressure of the aneurysm was attempted by hypothermic circulatory arrest of the extracorporeal circulation or by adenosine-induced transient cardiac stand still.3)4)9)18)33)43)
All patients underwent cerebral CT angiography and brain CT immediately after surgery. If not contraindicated, patients underwent conventional cerebral angiography within 1 week after surgery to determine parent artery patency, aneurysm obliteration, and residual sac.
Endovascular procedure (Fig. 2)
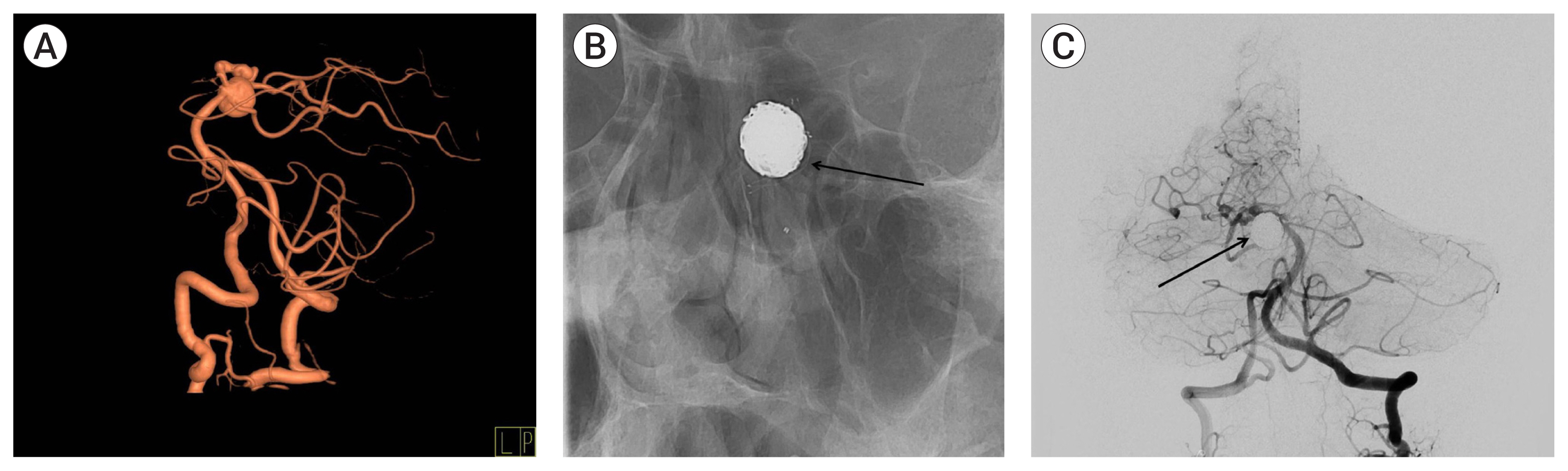
(A) Three dimensional reconstruction of conventional cerebral angiography showing a superior cerebellar artery aneurysm. (B) Simple skull x-ray showing stent material (black arrow). (C) Conventional cerebral angiography following coil embolization showing complete obliteration of the aneurysm with coil mass (black arrow).
All patients with unruptured aneurysms received dual antiplatelet medications (100 mg/day aspirin and 75 mg/day clopidogrel) for at least 7 days before the procedure. All procedures were performed under general anesthesia; during the procedure, heparin was administered to achieve a target activated clotting time (ACT) of 200–250 sec, or 2–2.5 times baseline. Dual antiplatelet treatment was continued for at least 3 months after treatment. Patients with ruptured aneurysms were not treated with antiplatelet agents before the procedure. After filling the first coil in the aneurysm, heparin was administered to the same target ACT. Because extraventricular drainage (EVD), ventriculoperitoneal shunt, or craniectomy may be required due to intracranial hemorrhage, hydrocephalus, or brain swelling after the procedure, the procedure was terminated without using a stent if possible. If there were no specific events, patients were not administered antiplatelet agents after the procedure. If stents were used, however, antiplatelet agents were administered after the procedure, as above. All patients underwent post-procedural MR angiography to determine the baseline for comparison with follow-up images and to determine the occurrence of acute cerebral infarction. During the study period, flow diverter stents were not implanted for posterior circulation aneurysms.
Clinical and radiologic outcomes and follow-up
Treatment outcomes were assessed clinically and radiographically. Clinical outcomes were assessed using the Glasgow outcome scale (GOS).15)22) Preoperative GOS scores were used to determine baseline neurological condition, and postoperative GOS scores were assessed 6 months after the operation. Cranial nerve palsy was also compared immediately after and 6 months after surgery. Radiologic outcomes were assessed based on imaging tests performed after microsurgery or endovascular treatment. In patients who underwent microsurgery, aneurysm obliteration was determined by brain CT and cerebral CT angiography immediately after surgery, followed by conventional cerebral angiography 1 week later, and cerebral CT angiography performed 6 months later and once yearly thereafter. In patients who underwent endovascular treatment, aneurysm obliteration was determined by MR angiography immediately after the procedure and brain CT 1 month later, and MR angiography was performed 6 months later and every 1–2 years thereafter. Patients who experienced new neurological symptoms regardless of follow-up period were assessed by brain CT and brain MR imaging to determine the occurrence of new complications, such as intracranial hemorrhage or cerebral infarction. If residual aneurysm growth or recurrence in the treated aneurysm was strongly suspected on serial cerebral CT or MR angiography of patients in either group, patients were evaluated by conventional four vessel cerebral angiography. Recurrence was defined as an increase in size of the residual lesion on follow-up imaging or contrast filling of the sac after complete obliteration (Fig. 3). If conventional cerebral angiography showed aneurysm recurrence or an increase in size of the residual aneurysm, retreatment was actively considered.

(A) Three dimensional reconstruction of conventional cerebral angiography showing a vertebral artery aneurysm. (B) Conventional cerebral angiography after embolization showing complete obliteration of the aneurysm. (C) Follow-up angiography showing recurrence due to coil compaction. (D) Conventional cerebral angiography after additional coiling, showing complete obliteration of the aneurysm.
Data collection and statistical analysis
Categorical variables in groups of patients were compared by chi-square or Fisher’s exact test, as appropriate, whereas continuous variables were compared by Mann-Whitney U-tests. Clinical outcomes in groups of patients were compared using the Wilcoxon signed-rank test. All statistical analyses were performed using a commercially available software package (GraphPad Instat7.0, GraphPad Software, San Diego, CA, USA), with p<0.05 considered statistically significant.
RESULTS
Characteristics of patients and aneurysms
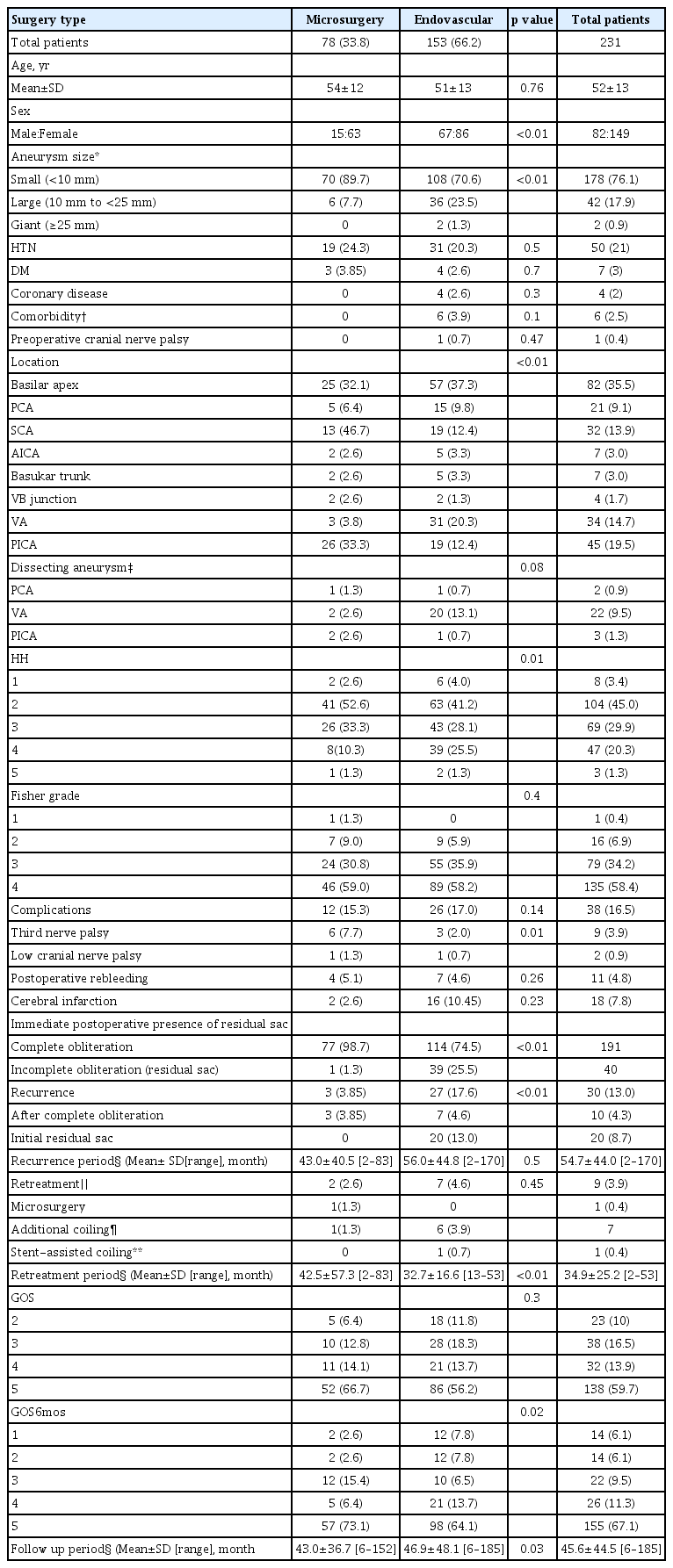
Of 621 aneurysms, 231 (37.2%) were ruptured and 390 (62.8%) were unruptured. A total of 187 aneurysms (30.1%), including 78 ruptured and 109 unruptured aneurysms, were treated with surgical clipping, and 434 (69.9%), including 153 ruptured and 281 unruptured aneurysms, were treated with endovascular coiling. Of the 231 ruptured aneurysms, 78 (22.5%) were treated microsurgically and 153 (66.2%) were treated with endovascular coiling, whereas, of the total 390 unruptured aneurysms, 109 (28.1%) were treated microsurgically and 281 (71.9%) were treated endovascularly.
The basilar apex was the most common anatomical location, accounting for 271 (43.6%) of the total 621 aneurysms followed by VA (n=94, 15.1%), SCA (n=91, 14.7%), PICA (n=87, 14.0%) and so on.
Dissecting cases were 28 and 27 for unruptured and ruptured aneurysms, respectively. The characteristics of the enrolled aneurysm are summarized in Tables 3 and 4. All other aneurysms were saccular aneurysm.
Demographic and clinical characteristics and treatment outcomes of patients with unruptured posterior circulation aneurysms
Unruptured posterior circulation aneurysm
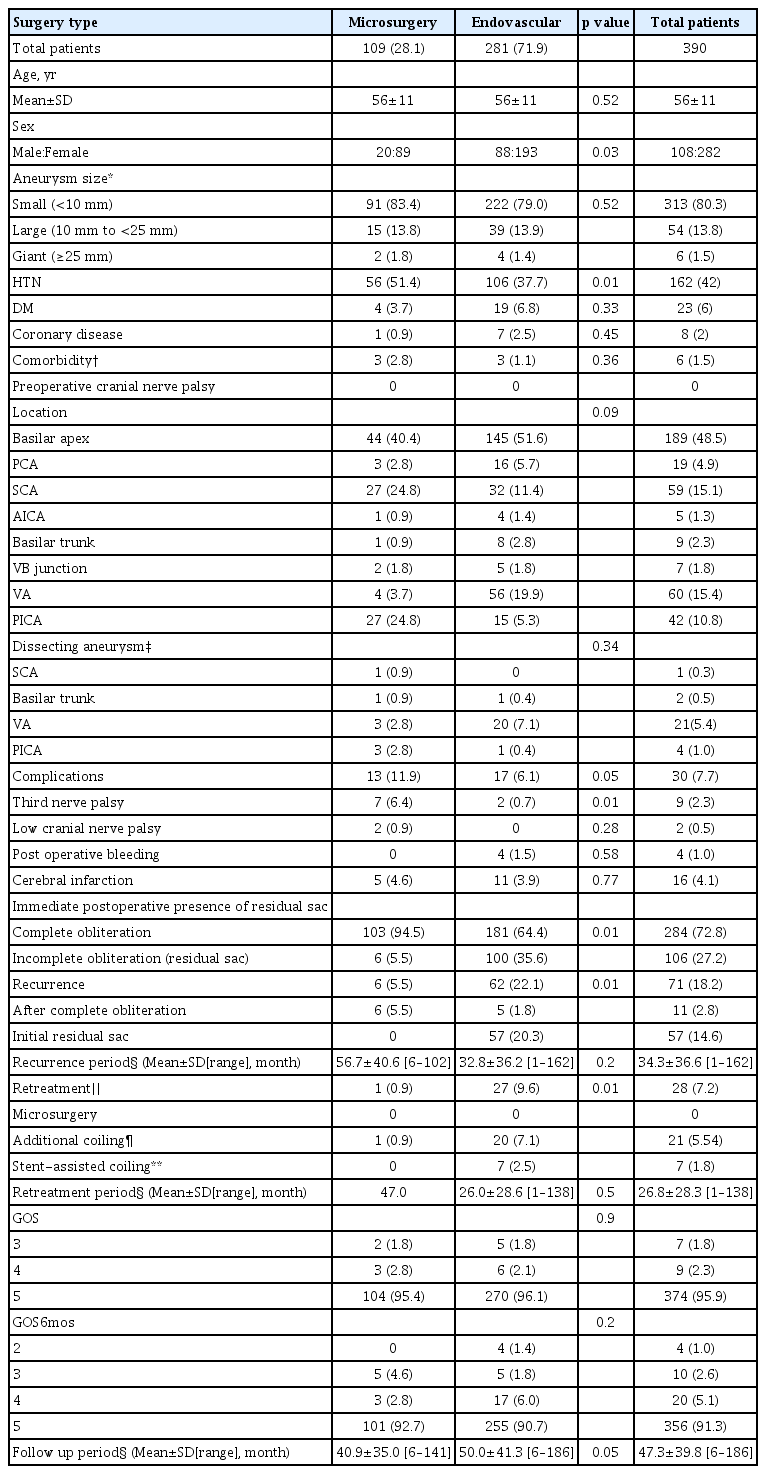
Characteristics and treatment outcomes of patients with unruptured posterior circulation aneurysms are summarized in Table 3. The mean ages of patients in both the microsurgery and endovascular treatment group were 56 years, with no significant difference between these two groups. A comparison of sex ratio showed greater female dominance in the microsurgery group (p=0.03). Aneurysm size and location did not differ significantly in these two groups. The rate of third nerve palsy was significantly higher in the microsurgery than in the endovascular treatment group (6.4% [7/109] vs. 0.7% [2/281], p=0.01). Six months later, the third nerve palsy improved in two patients in the microsurgery group and one in the endovascular treatment group. Rates of postoperative bleeding and cerebral infarction did not differ significantly in these two groups, nor did GOS scores. Immediate postoperative conventional cerebral angiography showed that the rate of residual lesions was significantly lower in the microsurgery than in the endovascular treatment group (5.5% [6/109] vs. 35.6% [100/281], p<0.01; Table 3). The mean follow up period for microsurgery and endovascular groups was 40.9 and 50 months, respectively.
Ruptured aneurysms
Characteristics and treatment outcomes of patients with ruptured posterior circulation aneurysms are summarized in Table 4. Of the 231 ruptured aneurysms, 78 (33.8%) were treated microsurgically and 153 (66.2%) were treated endovascularly. The mean ages of the patients in these two groups were 54 and 51 years, respectively. Significantly greater female dominance was observed in the microsurgery than in the endovascular group (p<0.01). Aneurysm size of aneurysm differed significantly in these two groups, with 24.8% of aneurysms treated endovascularly and 7.7% of those treated microsurgically being >10 mm in size (p<0.01). Aneurysm locations also differed significantly, with SCA and PICA aneurysms tending to be treated microsurgically, and VA aneurysm tending to be treated endovascularly. The rate of third nerve palsy was significantly higher in the microsurgery than in the endovascular treatment group (7.7% [6/78] vs. 2.0% [3/153], p=0.01). Six months later, the third nerve palsy improved in one patient in the microsurgery group and none in the endovascular treatment group. Also there was a case where bilateral abducens nerve palsy developed due to the ruptured VA aneurysm causing increased intracranial pressure crisis. However, this case was not included in the Table as the symptom was not worsened before and after the procedure. Although rates of postoperative bleeding did not differ significantly in these two groups, the rate of postoperative cerebral infarction tended to be lower in the microsurgery than in the endovascular treatment group (2.6% [2/78] vs. 10.5% [16/153], p=0.23). Immediate postoperative and 6 month follow-up GOS scores did not differ significantly in the two groups. The rate of residual sac was significantly lower in the microsurgery than in the endovascular treatment group (1.3% [1/78] vs. 25.5% [39/153], p<0.01). The mean follow up period for microsurgery and endovascular groups was 43.0 and 46.9 months, respectively.
Bypass surgery
A total of 12 bypass surgeries were involved in our study; 8 unruptured and 4 ruptured cases. The most common location was VA (n=4), followed by distal PICA (n=3), VA-PICA (n=2), distal PICA (n=3), basilar-SCA junction (n=1), basilar trunk (n=1). Bypass was mandatory in these cases for following reasons: 1) for protecting of branching vessel patency in VA and VA-PICA aneurysms; 2) parent artery patency in basilar trunk, distal PICA, PCA and SCA. All these cases were dissecting aneurysms.
Aneurysm recurrence and retreatment
Details of aneurysm recurrence and retreatment are summarized in Tables 3 and 4. Of the 621 aneurysms, 37 (6.0%) required retreatment due to recurrence. Ninety-eight aneurysms recurred, including 68 (69.4%) unruptured and 30 (30.6%) ruptured aneurysms, with 21 (21.4%) aneurysms recurring after complete obliteration of aneurysm and 77 (78.6%) showing an increase in size of the initial residual sac.
Of the 68 recurrences in the unruptured group, 62 had been treated endovascularly and six by microsurgery (p=0.01). All six recurrences in the microsurgery group and five in the endovascular group occurred immediately after complete aneurysm obliteration. The remaining 57 aneurysms treated endovascularly were residual, initial stage lesions. The rate of retreatment was also significantly lower in the microsurgery than in the endovascular treatment group (0.9% vs. 9.6%, p<0.01; Table 3).
Of the 30 ruptured aneurysms that recurred, 27 were treated endovascularly and three were treated microsurgically (p<0.01). All three recurrences in the microsurgery group and seven in the endovascular group occurred immediately after complete aneurysm obliteration, whereas the remaining 20 aneurysms treated endovascularly had initial residual sac. However, the percentages of ruptured aneurysms requiring retreatment did not differ significantly in the microsurgery (2.6%) and endovascular treatment (4.6%) groups (Table 4).
A total of 37 aneurysms in 24 patients required retreatment, with four patients requiring two retreatments each and four requiring three or more retreatments. The remaining 16 patients required one retreatment each.
DISCUSSION
Several large studies have reported that the outcomes of endovascular treatment were superior to those of microsurgical treatment for posterior circulation aneurysms. In the International Study of Unruptured Intracranial Aneurysm (ISUIA) study, microsurgery was associated with a high morbidity rate, whereas the International Subarachnoid Aneurysm Trial (ISAT) study reported better clinical outcomes after endovascular treatment.21)23)42) The BRAT found that endovascular coiling resulted in better outcomes than microsurgical clipping at 1 year follow-up.21) Moreover, endovascular treatment was reported superior in the treatment of basilar bifurcation aneurysms.17) Similarly, our study found that the rates of cranial nerve palsy (particularly the third nerve) in patients with both ruptured and unruptured aneurysms were higher in the microsurgery than in the endovascular treatment group. By contrast, ISAT included only 58 patients with posterior circulation aneurysm, or only 2.7% of the total of 2,143 patients, and did not compare clinical outcomes in patients with posterior and anterior circulation aneurysms or in aneurysms treated microsurgically and endovascularly. In the ISUIA study, posterior circulation aneurysms were associated with poorer outcomes by both clipping and coiling, and this study did not directly compare outcomes of the two treatment modalities.42) Moreover, only 51% of aneurysms treated endovascularly were completely obliterated, with 21% being partially occluded and 24% experiencing obliteration failure, indicating that endovascular outcomes in this study were not satisfactory.42)
Endovascular treatment has shown high rates of incomplete occlusion and recurrence during follow-up, with recurrence rates reported to range from 10% to 33%.13)28)29)32)35)37) Our study also showed higher recurrence and retreatment rates in the endovascular treatment group. In particular, endovascular treatment of ruptured aneurysms resulted in a residual sac rate of 25.5% and a retreatment rate of 4.6%. The proportion of ruptured aneurysms >10 mm in size was significantly higher in the endovascular treatment group (p<0.01), and the distribution of aneurysm location also differed significantly (p<0.01), factors that may have affected recurrence rates in the two groups. However, recurrence rates of both ruptured and unruptured aneurysms tended to be higher after endovascular than after microsurgical treatment.
The goal of aneurysm treatment is complete occlusion. The Cerebral Aneurysm Re-rupture After Treatment (CARAT) trial and other studies have reported that residual aneurysm is a direct risk for rupture.13)24) The CARAT study reported that the risk of rupture was lower for completely occluded aneurysms (1.1%) than for aneurysms occluded 70–90% (5.8%).13) Posterior circulation aneurysms require more definitive treatment than anterior circulation aneurysms because the former are more likely to rupture.42) In our study, the rates of residual lesions and need for retreatment were significantly higher in the endovascular treatment group. Although patient and aneurysm characteristics differed somewhat in the surgical and endovascular groups, the endovascular group was more often incomplete than the surgical group, with the former requiring retreatment. The limitations of this endovascular technique raise questions about the efficacy of endovascular treatment.
Third nerve palsy is common in aneurysms originating from the apex of the BA and the SCA projecting posteriorly.1)10)25)31) The two possible mechanisms for postoperative oculomotor dysfuntion are direct mechanical damange and brainstem infarction due to vascular complication. This study showed similar results to the previous studies.
In microsurgery group, whether ruptured or unruptured, third nerve palsy was commonly occurred immediately after surgery. The common location for this neurologic deficit was basilar apex and SCA. This is thought to be results from the process of approaching the aneurysm or protecting the parent artery. There were 3 cases of third nerve palsy in the endovascular treatment group. One case was due to the lateral medullary syndrome from the cerebral infarction caused during the coil embolization of ruptured VA aneurysm. The other 2 cases were coil embolization of BA and SCA aneurysms causing coil mass compressing the third nerve.
Procedure related low cranial nerve deficits were observed in 4 cases. Among these patients, 3 cases were related to microsurgery during the treatment of unruptured AICA, VA-PICA junction or ruptured distal PICA aneurysms. The other case was ruptured VA aneurysm causing 9th and 10th nerve palsy due to a lateral medullary syndrome, which was successfully treated by stent assisted coil embolization.
In the case of low cranial nerve, there were 4 cases in total patients, 3 cases occurred immediately after treatment of AICA, VA-PICA junction, distal PICA aneurysm with microsurgery. In one case, after stent assisted coil embolization for ruptured VA aneurysm, 9th, 10th nerve palsy occurred due to lateral medullary syndrome.
Flow diverter stents are increasingly used in the treatment of both posterior and anterior circulation aneurysms. Complete aneurysm obliteration rates ranged from 69% to 100%, and permanent morbidity and mortality rates from 1% to 15%. Flow diverters are indicated for anterior circulation aneurysms, especially paraclinoid aneurysms, because of easier access and the absence of a perforator. However, risks of ischemic complications are higher in posterior circulation aneurysms, such as those in the vertebral and basilar arteries, because of the presence of perforators and branches to the brainstem.6)7)14)19)20)36)40) Although relatively little is known about the use of flow diverters for posterior circulation aneurysm, these patients tend to be at higher risk of ischemic stroke than those with anterior circulation aneurysms. Further studies are needed to assess the stability and effectiveness of flow diverters in the treatment of posterior circulation aneurysms.
Advancement of various endovascular tools such as wires, microcatheters, guiding catheters, stents, coils and flow diverters helped to expand the indications of endovascular treatment. The combination of this modality improvement with experienced neurointerventionists resulted in a favorable clinical outcome. On the contrary, the indications for microsurgery are gradually narrowing. Our study corroborated this results too as comparing the number of adopted modalities by year (Fig. 4). Nevertheless, microsurgery remains a competitive, primary therapy for SCA, PCA, distal AICA, and PICA aneurysms.32) Because endovascular treatment often fails, microsurgery is a better treatment modality for complex aneurysms, such as those of large size, wide neck, and dysmorphic branches.16)32) In addition, there are cases where microsurgery is essential, such as complex aneurysms where bypass technique is mandatory. In the present study, outcomes did not differ significantly in the microsurgery and endovascular groups. Although not statistically significant, rates of post-treatment bleeding and cerebral infarction were higher in the endovascular group.
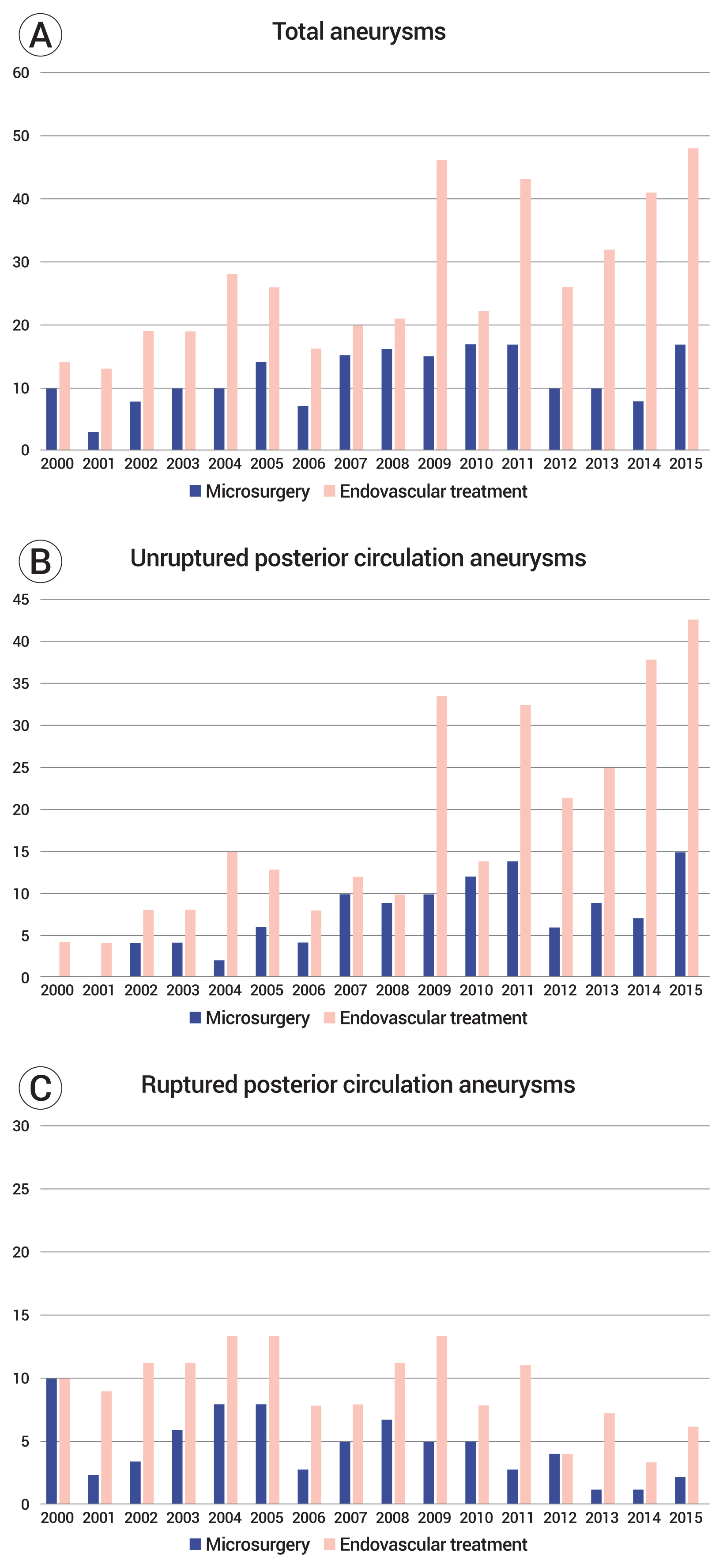
(A) Number of microsurgery and endovascular treatment groups by year in total aneurysms. (B) Number of microsurgery and endovascular treatment groups by year in unruptured aneurysms. (C) Number of microsurgery and endovascular treatment groups by year in ruptured aneurysms.
The reported superiority of endovascular treatment in the treatment of posterior circulation aneurysms is not based on evidence, such as complete occlusion rate or neurological outcome. Rather, it is regarded as superior because of the technical difficulties of microsurgery and the burden of postoperative complication. This study was not performed to emphasize the advantages and superiority of microsurgery. Rather, this is to remind that there are still areas in which microsurgery is needed in an era where endovascular treatment is a priority in the treatment of posterior circulation aneurysms. These results may help in the future treatment of posterior circulation aneurysms.
Limitations
The present study had several limitations, including its retrospective design. Moreover, there was an inherent selection bias because of the different skill sets and preferences of the cerebrovascular team, which included two neurosurgeons and two neurointerventionists. Typically, clinicians tend to use treatment methods with which they are more familiar. In addition, it was difficult to accurately compare rates of aneurysm occlusion and complications because aneurysm locations differed in the microsurgery and endovascular treatment groups. In addition, ruptured aneurysms >10 mm in size more frequently underwent endovascular treatment, a difference that was statistically significant. Thus, the higher recurrence rate in the endovascular treatment group may have been due to the larger aneurysm size in that group. Another limitation may have been due to the definition of residual lesions after treatment, which was based on CT angiography, MR angiography, or conventional cerebral angiography. Because imaging modalities used to detect residual lesions after treatment have not been standardized, cerebral angiography may have detected more residual lesions. Furthermore, although flow diverter stents are increasingly used in patients with aneurysms, they were excluded when this study was planned because they were difficult to use.
CONCLUSIONS
Endovascular treatment has increasingly become an alternative modality for microsurgery in posterior circulation aneurysm, whereas the indication for microsurgery is greatly reduced. However, the absolute number of microsurgery is maintained showing that it is a still valuable technique, as advances in endovascular or stent-assisted coiling have not solved many of the challenges inherent in the management of complex aneurysms. Hence, the advantages and limitations of both modalities must be carefully concerned in posterior circulation aneurysm to obtain favorable outcome.
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.