 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 19(1); 2017 > Article |
|
Abstract
Objective
Ruptured middle cerebral artery (MCA) aneurysm with intrasylvian hematoma usually accompanied by progressive cerebral swelling with poorer outcomes. The authors present characteristics and importance of intrasylvian hematoma removal in the aneurysm surgery.
Materials and Methods
From 2012 February to 2014 March, 24 aneurysm surgeries for ruptured MCA aneurysms with intrasylvian hematoma were performed in the authors' clinic. The patients were classified according to three groups. Group A included patients who underwent decompressive craniectomy within a few days after aneurysm surgery due to progressive cerebral swelling, group B included patients for whom decompression was not necessary, and group C included patients who showed severe cerebral swelling on admission and decompressive craniectomy and aneurysm surgery in one stage.
Results
The mean hematoma volume on admission was 28.56 mL, 24.96 mL, and 66.78 mL for groups A, B and C, respectively. Removal of a larger amount of hematoma was observed on postoperative computerized tomography scan in groups B and C (63.2% and 59.0%) compared with group A (33.4%). Although no statistical difference was found between group A and group B (p = 0.115), it tends to show the lesser amount of hematoma removed, the more likely cerebral swelling will progress.
Conclusion
The lesser amount of hematoma in ruptured MCA aneurysm with intrasylvian hematoma tends to show benign clinical course than larger amounts. But, even if the hematoma is not easily removed in the operation, we suggest the other procedures such as continuous external catheter drainage of hematoma to avoid unnecessary coagulation or brain retraction.
Outcomes of ruptured middle cerebral artery (MCA) aneurysm are related to the presence of intracerebral hemorrhage (ICH), amounts of subarachnoid hemorrhage (SAH), and brain swelling. Some studies have reported on prediction of the prognosis from three variables, and described the clinical characteristics. Midline shifting on computerized tomography (CT) scan showing increased intracranial pressure (ICP) and severe brain swelling was highly associated with poorer outcomes. These reports mentioned the usefulness of early surgical clipping and evacuation of hematoma because dramatic improvement could be possible, as seen in the operation from traumatic epidural hematoma (EDH).5)9)10)
The CT findings of SAH with intrasylvian hematoma show specifically that the center of hematoma starts from the Sylvian fissure, extended pushing frontal and/or temporal lobe, and widening of the fissure due to successive thick hematoma itself. The amount of hematoma is generally smaller than that of solitary temporal hematoma without SAH, and only a few patients show midline shifting on CT scan. However, the clinical course is very unpredictable. Ruptured MCA aneurysm with intrasylvian hematoma shows particularly rapid progression of cerebral swelling and in many cases decompressive craniectomy may be necessary within a few days from aneurysm surgery. Intrasylvian hematoma is different from solitary temporal ICH of MCA aneurysm in that it cannot be easily removed. Because the hematoma is very sticky like brain tumor, many neurosurgeons have difficulty in aspiration and removing it using an aspiration maneuver. Such manipulation to remove the sticky hematoma can aggravate cerebral swelling secondarily, after the operation. The authors postulated that such remaining hematoma is related to progression of cerebral swelling and neurological deterioration.
The authors analyzed 24 ruptured MCA aneurysm patients with intrasylvain hematomas, and classified for three groups as patients who underwent decompressive craniectomy within a few days after aneurysm surgery, well treated patients without further operation, and patients who underwent decompressive craniectomy and surgical clipping in one stage due to severe cerebral swelling. Clinical characteristics and CT findings from each group were studied.
From 2012 February to 2014 March, surgical clipping of 24 patients of ruptured MCA aneurysms with intrasylvian hematoma was performed in the author's clinic. CT findings and clinical features from the patients' medical records were analyzed retrospectively. This study was approved by the Institutional Review Board of the authors' institute. Intrasylvian hematoma was defined as follows: 1) hematoma should begin from a ruptured MCA aneurysm in the Sylvian fissure, 2) hematoma extends outside pushing frontal and/or temporal cortex, 3) amounts of hematoma volume can be calculated at least above 10 mL on CT scan. Hematoma volume less than 10 mL or that could not form hematoma but only showed dense SAH was excluded in this study. Hematoma volume was calculated using the methods used by Kothari et al. as the formula a ├Ś b ├Ś c / 2 (a: the longest hemorrhage diameter by CT, b: the diameter 90 degrees to diameter b, c: the approximate number of CT slices with hemorrhage multiplied by the slice thickness).6)
The 24 patients who satisfied these radiographic conditions were then categorized as three groups. Group A (seven patients) included patients in whom decompressive craniectomy had to be performed within a few days after the first surgical clipping because brain swelling had become more aggravated. None of the patients showed rebleeding from a clipped aneurysm. However, in three patients, the hematoma volume was increased compared with the immediate postoperative CT scan. Group B included patients for whom a second operation was not necessary, and were treated conservatively after clipping. Group C included four patients who showed the poorest initial neurological status (Hunt-Hess Grade IV or V), and severe brain swelling such as uncal herniation on CT scan. From the first choice of treatment, wide bone flap removal and clipping of aneurysms were scheduled.
Postoperative CT scans were obtained from all patients, and remaining hematoma volume was calculated using the same formula a ├Ś b ├Ś c / 2. If the hematoma volume was so small that calculation was impossible or less than 10 mL, the amount of volume was designated as zero. Removal ratio of hematoma was calculated as follows: (initial volume of hematoma - postoperative volume of hematoma) / initial volume of hematoma. If most of the hematoma was removed, and almost no hematoma could be seen, the removal ratio was designated as 100%. In contrast, if there was no change of hematoma volume between initial and immediate postoperative CT scan, the value was designated as zero. Clinical outcome at 90 days was evaluated using the Glasgow outcome scale (GOS) like most clinical trials or analysis from the medical reports.3)
Statistical analyses were performed using the unpaired t-test using SPSS 13.0 (SPSS Inc., Chicago, IL, USA), and p-values less than 0.05 were considered statistically significant.
In 24 patients, male was dominant (male : female = 13 : 11). The mean age was 49.71 ┬▒ 4.350 and 49.38 ┬▒ 9.963 years in Group A and Group B, respectively. The mean age of Group C (54.50 ┬▒ 11.733 years) was higher than that of the other groups, however, statistical difference was not found in the three groups (p = 0.894) (Table 1).
Favorable grade SAH (Hunt-Hess grade I to III) was observed in 57.1% of patients in group A, and 69.2% of patients in group B. In group C, all patients were in poor grade SAH (Hunt-Hess grade IV or V) on admission, and emergent decompressive craniectomy, removal of hematoma, and clipping of aneurysm were performed in one stage.
In group A, seven patients had undergone decompressive craniectomy after surgical clipping within a few days. The mean volume of hematoma on initial CT scan was 28.56 ┬▒ 7.716 mL, similar to that of Group B (24.96 ┬▒ 19.989 mL), but less than 66.78 ┬▒ 21.101 mL of Group C (p = 0.015). The mean removal ratio of hematoma was 33.4% in group A. From postoperative day (POD) 1 to POD 6, neurological deterioration and progression of cerebral swelling was observed on CT scan and emergent decompressive craniectomy was performed. Two patients underwent the operation on POD 1, two on POD 2, two on POD 5, and one patient on POD 6. Three patients showed marked expansion of hematoma and cerebral swelling on CT scan. However, bleeding from clipped aneurysm was not found on the operating field. Three of seven patients showed good recovery (GOS 4 or 5), but two patients expired (Table 2).
In group B, 13 of 24 patients (54.1%) could be treated with surgical clipping only without additional decompressive craniectomy. The mean removal ratio of hematoma (63.2%) was higher than in Group A (33.4%), however, statistical difference was not observed (p = 0.115). Almost complete removal of hematoma was observed on immediate postoperative CT scan in six of 13 patients (46.1%). In those patients, seven patients (53.8%) showed good recovery without neurological deficit (GOS 5), however, two patients expired from pulmonary complication and sepsis, respectively, in the intensive care unit.
Group C was composed of four patients who showed large amounts of hematoma and severe brain swelling. All patients were in poor grade SAH of Hunt-Hess grade 4 or 5. In the preoperative stage, prophylactic decompressive craniectomy was planned, and wide craniectomy was performed. Although two of four patients expired, the rest showed neurological improvement to GOS 3 during 3 months period hospitalization. Three patients showed large amounts of hematoma more than 60 mL.
A 44-year-old male visited emergency room provoking a severe headache after sudden loss of consciousness. Initial consciousness level was deep drowsy (Hunt-Hess 3). Brain CT showed a typical SAH from MCA aneurysmal rupture in that SAH was mainly dispersed prominently along the left Sylvian fissure. Intrasylvian hematoma volume was calculated from the formula ABC/2 as 17.3 mL (Fig. 1A, B). Routine craniotomy and surgical clipping using medial transsylvian approach was performed after drainage of lumbar cerebrospinal fluid (CSF) to slag down the brain. Intrasylvian hematoma was very sticky, and could not be removed by simple suction and irrigation. Surgical clipping was performed successfully on ruptured aneurysm only and there was no complication during the procedure. But, sufficient hematoma removal was not found on immediate postoperative CT scans, and the removal ratio of hematoma was considered as zero (Fig. 1C). On POD 3, the declination of consciousness was observed, and aggravated cerebral swelling around residual hematoma was found on CT scan (Fig. 1D). Midline shifting was noticed and emergent decompressive craniectomy was performed to reduce the high intracranial pressure (ICP). The patient was discharged with some neurological disability (GOS 3) after 3 months.
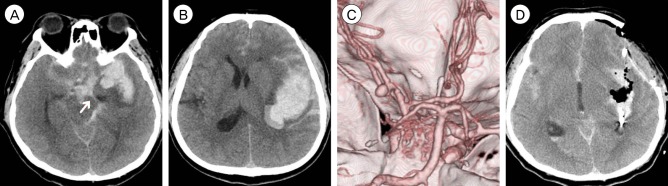
A 42-year-old male was transferred to emergency room presenting with semicomatous consciousness (Hunt-Hess 5). On neurological examination, the decorticate posture was noted, and the left pupil was unresponsive to light reflex with 5 mm size. Brain CT and CT angiography revealed SAH from ruptured MCA aneurysm and large amounts of intrasylvian hematoma about 64.9 mL (Fig. 2). Emergent decompressive craniectomy and surgical clipping was performed in one stage. Surgical clipping was performed in routine manners after lumbar drainage, and the large amounts of hematoma was removed by simple suction. Postoperative brain CT revealed there was little residual hematoma, and removal ratio of hematoma was calculated as 100%. The patient showed neurological improvement gradually during hospitalization, consciousness fully recovered, and discharged with some neurological deficit after 3 months hospitalization and rehabilitation treatment (GOS 3).
In patients who underwent clipping surgery in MCA aneurysms with intrasylvian hematoma, prediction of clinical course is difficult because some patients persist doing well against vasospasm and cerebral swelling but others do not. Even though patients' condition may be favorable after aneurysm surgery, the successive neurological deterioration resulting from progression of cerebral swelling can be observed within a few days. To study factors regarding progression of swelling, the authors postulated that remaining hematoma after surgery may play an important role in the process. These start from the authors' experiences that common CT findings of patients who showed progression of swelling and underwent decompressive craniectomy finally revealed a large remaining thick intrasylvian hematoma on immediate postoperative CT scan.
Initial neurological statue of the patients was associated with the clinical course after first aneurysm surgery. For example, the five patients of 13 good grade SAH of Hunt-Hess grade 1-3 showed progressive cerebral swelling and surgical decompression within POD 3 days. The seven patients of eleven of Hunt-Hess 4 or 5 grades required the decompressive craniectomy in one stage or within POD 3 days. This findings was statistically significant (p < 0.05)
The most characteristic feature of ruptured MCA aneurysm different from those of other locations is the formation of temporal ICH. The pathogenesis of ICH formation is explained from adhesion of a ruptured aneurysm dome to the pia mater and the rapid obstruction of the subarachnoid space by dense arachnoid, fibrin, and blood clot. Even complete obstruction can cause pure temporal ICH without SAH expressed as Fisher Grade IV.2) Partial obstruction of the subarachnoid space and subarachnoid clot entrapped in the relatively larger Sylvian fissure has been known as a mechanism of intrasylvian hematoma formation.1)7)
Poor outcome of patients with intrasylvian hematoma has been reported, and removal of the intrasylvian hematoma is very difficult according to Saito et al.8)9)10) Although to the best of our knowledge, there has been no report on measurement of removal ratio, the mean ratio of hematoma removal in group A was 33.4% in the authors' study. Statistical difference was absent from lower sample size, it was much smaller than 63.2% in group B. There was not much difference in initial hematoma volume on CT scan (28.6 mL for group A, and 25 mL for group B). Interestingly, three patients showed progression of hematoma without evidence of rebleeding from a clipped aneurysm. This is why we concluded that remaining hematoma itself is a major triggering factor for progression of cerebral swelling. Removal of hematoma in case 2 was relatively easier than case 1. Massive hematoma enabled simple suction procedure in operation. In contrast, it was impossible because of arachnoid adhesion and sticky hematoma in case 2. Brain retraction to remove hematoma and small vein coagulation for bleeding control would aggravate progression of secondary cerebral brain swelling on POD 2.
According to Jickling et al., a delayed hemorrhagic transformation occurs after 18 to 24 hours of stroke onset for several weeks, and the mechanism contributes to the processes involved in delayed blood-brain-barrier (BBB) opening after stroke.4) Removal of the blood clot in the intrasylvian hematoma is very difficult. Unlike hypertensive ICH that results from small lenticulostriate arteries and forms the global hematoma capsule and homogeneous character, intrasylvian hematoma consists of blood clot, thick arachnid trabecular, fibrin, and brain debrides. It is impossible to remove by simple aspiration, and, sometimes, coagulation and removal with brain retraction may be necessary. These maneuvers may cause secondary damage to small vessels.12) In addition, the venous infarction from this may be the cause of venous infarction, progression of swelling, and even hemorrhagic transformation. The small vessel injury by coagulation and mechanical injury from brain retraction would cause infarction and following secondary brain swelling in the period.
If the remnant hematoma is the main cause of progression of cerebral swelling and surgical removal is very difficult, we suggest that the next step is the prophylactic decompressive craniectomy. Group C, composed of patients who underwent aneurysm surgery and decompression in one stage did not show a good clinical result, but it is due to poor initial status. In case illustration 2, the semicomatous patient with uncal herniation showed neurological improvement. It means even in poor grade SAH with large sylvian hematoma patient, aggressive hematoma removal and surgical decompression is necessary in the patients. Smith et al. reported that prophylactic decompressive craniectomy in SAH patients with large intrasylvian hemaotmas can be performed safely.11)
The limitation of this study is lack of statistical difference due to the small sample size. However, the finding that patients in whom the hematoma could not be removed efficiently showed progression of cerebral swelling, although initial hematoma volume was similar, provide neurosurgeons with some important information.
This study suggests that the lesser amounts of hematoma that were removed on the first aneurysm surgery in MCA aneurysm with intrasylvian hematoma, the higher rates of progression of cerebral swelling and decompressive craniectomy may be considered. In addition, it shows that the ratio of hematoma removal can be an important prognostic factor. In cases with large hematoma, we recommend maximal removal of hematoma with aspiration as possible in the range of minima cortical and vessel injury; but, if the hematoma volume is smaller, cautious removal of the blood clot without injuring the vessels or avoidance of stronger brain retraction. If a very small blood clot is removed, and the brain is not sunken, cautiously consider prophylactic decompressive craniectomy in poor grade patients.
References
1. Ba┼¤kaya MK, Menendez JA, Y├╝ceer N, Polin RS, Nanda A. Results of surgical treatment of intrasylvian hematomas due to ruptured intracranial aneurysms. Clin Neurol Neurosurg. 2011 4;103(1):23-28.

2. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980 1;6(1):1-9;


3. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow outcome scale. J Neurol Neurosurg Psychiatry. 1981 4;44(4):285-293;



4. Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014 2;34(2):185-199;


5. Kopera M, Majchrzak H, Kaspera W. Prognostic factors in patients with intracerebral hematoma caused by ruptured middle cerebral artery aneurysm. Neurol Neurochir Pol. 1999 Mar-Apr;33(2):389-401;

6. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996 8;27(8):1304-1305;


7. Pasqualin A, Bazzan A, Cavazzani P, Scienza R, Licata C, Da Pian R. Intracranial hematomas following aneurysmal rupture: experience with 309 cases. Surg Neurol. 1986 1;25(1):6-17;


8. Saito A, Akamatsu Y, Mikawa S, Sugawara T, Seki H. Comparison of large intrasylvian and subpial hematomas caused by rupture of middle cerebral artery aneurysm. Neurol Med Chir (Tokyo). 2010 50(4):281-285;


9. Shim YS, Moon CT, Chun YI, Koh YC. Grading of intracerebral hemorrhage in ruptured middle cerebral artery aneurysms. J Korean Neurosurg Soc. 2012 5;51(5):268-271;




10. Shimoda M, Oda S, Mamata Y, Tsugane R, Sato O. Surgical indications in patients with an intracerebral hemorrhage due to ruptured middle cerebral artery aneurysm. J Neurosurg. 1997 8;87(2):170-175;


Fig.┬Ā1
CT and CT angiography in case 1. (A, B) Initial CT scans reveal characteristic findings of ruptured MCA aneurysm with intrasylvian hematoma. Hematoma volume was calculated as 17.6 mL. (C) Immediate postoperative CT finding of residual intrasylvian hematoma not sufficiently removed during aneurysm surgery. (D) Aggravated perihematoma swelling with midline sifting on POD 3. CT = computed tomography; MCA = middle cerebral artery; POD = post-operative day.
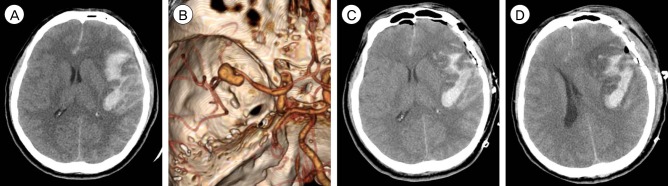
Fig.┬Ā2
CT and CT angiography in case 2. (A-C) SAH and intrasylvian hematoma from ruptured MCA aneurysm calculated as 64.9 mL. The arrow indicates uncal herniation. (D) Immediate postoperative CT reveals near total removal of intraysylvian hematoma. CT = computed tomography; SAH = subarachnoid hemorrhage; MCA = middle cerebral artery.
Table┬Ā1
Clinical features in 24 ruptured MCA aneurysms with intrasylvian hematomas on admission (number of patients)
MCA = middle cerebral artery; SD = standard deviation.
Group A = patients who underwent decompressive craniectomy within a few days after aneurysm surgery; Group B = patients who were treated conservatively after aneurysm surgery; Group C = patients who underwent surgical clipping and decompressive craniectomy in one stage
Table┬Ā2
Clinical outcomes (90 days GOS) and mean ratio of hematoma removal
- TOOLS
-
METRICS

-
- 6 Crossref
- 0 Scopus
- 2,481 View
- 25 Download
- Related articles
-
Surgical Experience of Distal Middle Cerebral Artery Aneurysm Rupture.2009 June;11(2)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



