Spontaneous Regression of Aneurysm Remnant after Incomplete Surgical Clipping in a Patient with Ruptured Cerebral Aneurysm
Article information
Abstract
Cases of spontaneous regression of cerebral aneurysm remnant after incomplete surgical clipping have been rarely reported. This paper reports the regression of an aneurysm remnant after incomplete surgical clipping during postsurgical follow-up. A 50-year-old male presented with subarachnoid hemorrhage because of rupture of an anterior communicating artery aneurysm. An emergency clipping of the aneurysm was performed. A cerebral angiography, which was performed two weeks postoperatively, revealed an aneurysm remnant. The patient refused additional treatment and was discharged without apparent neurological deficit. One-year follow up cerebral angiography demonstrated a partially regressed aneurysm remnant.
INTRODUCTION
Cerebral aneurysm remnants are major concerns after incomplete microsurgical clipping. The risk of regrowth and rupture of a remnant aneurysm warrants close follow-up with digital subtraction angiography (DSA) with additional endovascular coiling or surgical clipping if needed.1)5)6) To the best of my knowledge, cases of spontaneous regression of aneurysm remnant after surgical clipping have rarely been reported. Here in, we report a rare case of aneurysm remnant which regressed without additional treatment during the observation period.
CASE REPORT
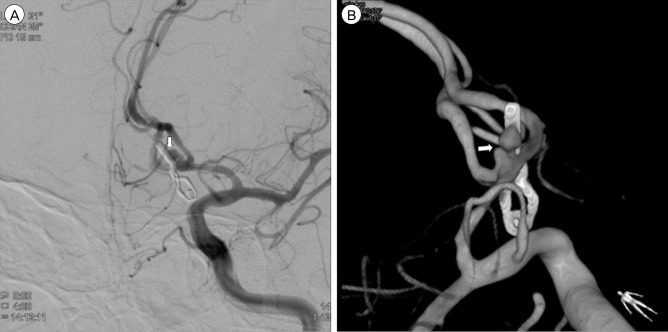
A 50-year-old male presented with sudden headache. At presentation, his Glasgow coma scale was 14 and no lateralizing sign was found. Brain computed tomography (CT) showed a thick subarachnoid hemorrhage in the basal cistern and a hemorrhage on the left frontal lobe and both lateral ventricles. CT angiography revealed an aneurysm at the anterior communicating artery (ACoA). The patient underwent preoperative digital subtraction angiography, which demonstrated a wide necked and bilobulated aneurysm with maximal size of 5.2 mm arising from the left ACoA (Fig. 1). The morphology of the aneurysm in this patient was deemed not amenable to treatment with coil embolization. An emergency clipping of the ruptured ACoA aneurysm was performed. Left side pterional craniotomy was chosen for aneurysm dome avoidance and more favorable view of the aneurysm neck. Brain swelling was moderate and ventricular drain was inserted through Payne's point. Ruptured point of the aneurysm was obliterated by a bayonet type clip. Further dissection of the aneurysm and confirmation of distal tip of the clip was difficult due to the gradually swelling brain and tight adhesions with the surrounding tissues. The patient tolerated the surgery well and showed no neurologic deterioration after surgery. Transcranial Doppler ultrasonography revealed no evidence of vasospasm. The patient's mental status fully recovered with conservative management. Follow-up cerebral angiography, which was obtained on the fourteenth postoperative day showed remnant aneurysm behind the surgical clip (Fig. 2). We recommended repositioning of the clip, but the patient was reluctant to undergo a reoperation. The patient was discharged with no apparent neurological deficit. Follow-up cerebral angiography at 12 months postoperatively showed the remnant aneurysm had regressed almost completely (Fig. 3). A recommendation to monitor the remaining tiny aneurysm with DSA after 12 months was made.
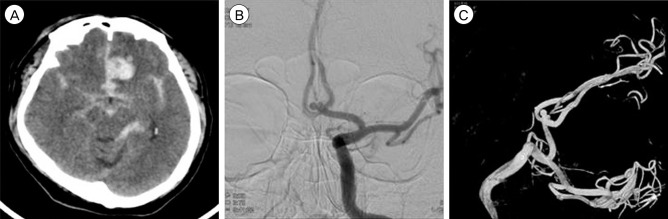
Brain computed tomography demonstrated subarachnoid hemorrhage in the basal cistern and a hematoma on the left frontal lobe. (A) Subsequent digital subtraction angiography revealed a ruptured anterior communicating artery aneurysm. (B) Left anteroposterior view. (C) 3D reconstruction.
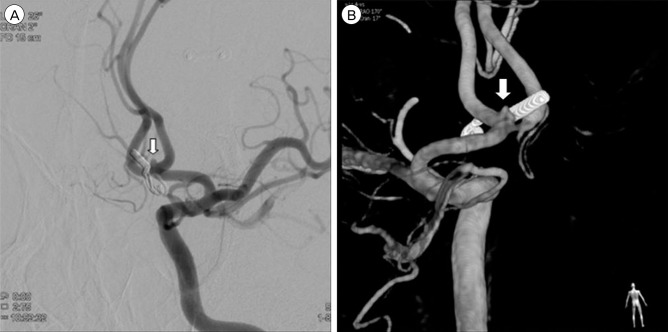
Digital subtraction angiography obtained two weeks after surgery showed remnant aneurysm behind the clip (arrow). (A) Left anterior oblique view. (B) 3D reconstruction.
DISCUSSION
According to previous research, the incidence of residual aneurysm after endovascular or surgical treatment ranged from 3.5% to 13%.1)4)5)6)8)11)12)14) The persistence of aneurysm remnants after surgical clipping may result from technical or anatomic difficulties at the time of surgery, slippage of a successfully placed clip, or regrowth of an aneurysmal sac from an incompletely excluded neck.15)16)17)19)20) Although the natural history of remnant aneurysms after surgical treatment has not been fully described, several studies have reported that aneurysm remnants were associated with persistent risk of rebleeding.12)13)15)16) Aneurysm remnants may also grow over time and can later produce symptoms by compressing neighboring structures.1)5)6)8)11) The reported incidence of enlargement and rebleeding rate in incompletely clipped aneurysms were 2.5-3.8% and 1.9-3.8, respectively.3)7) Although rare, cases of spontaneous obliteration of aneurysm remnant after surgical clipping of ruptured cerebral aneurysm have been reported.2)9)18) The incidence of aneurysm remnant was 3.8% in a series of 715 aneurysms that were managed using microsurgical techniques. Of 28 aneurysm remnants, 5 aneurysms disappeared in follow-up angiography.7) Most of the aneurysms that spontaneously disappeared had small diameters (less than 4 mm).
Several mechanisms and dynamic parameters related to spontaneous thrombosis of intracranial aneurysms have been suggested10); including 1) stasis of blood in the aneurysm sac, 2) a ratio of aneurysm sac volume to orifice area than 28:1 (mm3:mm2), 3) stagnation of blood in a large aneurysm, 4) endothelial injury due to turbulent blood flow, and 5) stasis of contrast material in the aneurysm sac. Hamilton and Dold proposed that hypotension, severe vasospasm, use of antifibrinolytic agents, giant aneurysm, and local injury to the arterial wall were associated with spontaneous obliteration of aneurysm in the ruptured aneurysms.9) Tan et al. reported a case of growth and spontaneous regression of a remnant aneurysm after incomplete surgical clipping of a ruptured middle cerebral artery aneurysm.18) In this case, the authors suggested that the sudden regression of the remnant aneurysm could be explained by the fact that endothelial repair exceeded endothelial destruction. They also suggested that thrombogenesis within the aneurysmal sac may have obliterated the aneurysm.
CT or magnetic resonance image may be helpful in documenting thrombus formation in the aneurysm sac. However, difficulties may be encountered in identifying the thrombus in a clipped aneurysm because of clip artifact. In our case, the patient had no medical factors such as dehydration or medical disease to accelerate thrombus formation within the aneurysm. Furthermore, the size of the aneurysm remnant was neither large or giant nor small (less than 4 mm). Although the exact mechanism involved in regression of aneurysm is unclear in our case, we speculated that the intraoperative manipulation may have led to endothelial injury that resulted in thrombus formation by platelet deposition and aggregation. In addition, vascular remodeling and stagnated blood flow within the aneurysm induced by the resulting vertically narrowed aneurysm neck after the incomplete clipping may have facilitated the formation of the thrombus.
At present, there is no reliable method to predict whether an aneurysm remnant will spontaneously regress or grow over time. Consequently, close follow-up with angiographic imaging is required in patients with incompletely clipped aneurysm, even in cases with spontaneously regressed aneurysm due to the possibility of recanalization in the future.
CONCLUSION
A case of spontaneous regression of cerebral aneurysm remnant after incomplete surgical clipping was reported. Close follow-up with angiographic imaging is required in incompletely clipped case.
Notes
Disclosure: The authors report no conflict of interest concerning the materials or methods in this study or the fundings specified in this paper.