The Efficacy of Single Barrel Superficial Temporal Artery-middle Cerebral Artery Bypass in Treatment of Adult Patients with Ischemic-type Moyamoya Disease
Article information
Abstract
Objective
So far, there is no study answering the question of which type of surgical technique is practically the most useful in the treatment of adult patients with ischemic type moyamoya disease (MMD). We evaluated the efficacy of single barrel superficial temporal artery (STA)-middle cerebral artery (MCA) bypass in the treatment of adult patients with ischemic type MMD by retrospectively collecting clinical and radiological data.
Materials and Methods
A retrospective review identified 31 adult patients who underwent 43 single barrel STA-MCA bypass procedures performed for treatment of ischemic-type MMD between 2006 and 2014. The male to female ratio was 17:14 and the mean age was 41 years (range, 21-65 years). Peri-operative complications, angiographic and clinical outcomes were analyzed retrospectively.
Results
The permanent neurological morbidity and mortality rates were 2.3% and 0%, respectively. During the observation period of a mean of 35 months (range, 12-73 months), 29 patients (93.5%) had no further cerebrovascular events and transient ischemic attack occurred in two patients (6.5%), resulting in an annual stroke risk of 2.2%. Follow-up computed tomography perfusion (CTP) (mean, 18.4 months after surgery) documented improved cerebral hemodynamics in the revascularized hemispheres (p < 0.001). Post-operative patency was clearly verified in 38 bypasses (88.4%) of 43 bypasses on follow-up imaging (mean, 16.5 months).
Conclusion
Our results suggest that single barrel STA-MCA bypass with wide dural opening is safe and durable method of cerebral revascularization in adult patients with ischemic type MMD and can be considered as a potential treatment option for adult patients with ischemic type MMD.
INTRODUCTION
The symptoms and signs of moyamoya disease (MMD) can be classified into 2 major etiological categories: those caused by brain ischemia (ischemic type) and those caused by the deleterious consequences of the compensatory fragile collateral vessels (hemorrhagic type). Clinically, symptoms of brain ischemia are usually found in MMD children, while transient or permanent brain infarction and intracranial hemorrhage are noted in MMD adults.7)9) In previous reports, it is 1. Characteristics of patients well recognized that surgical revascularization using direct and/or indirect bypass provides an improved outcome in patients presenting with the ischemic type.3)8) However, there are limited reports on the surgical outcome in patients with hemorrhagic MMD.18) Indirect revascularization surgeries, such as encephaloduroarteriosynangiosis and other variants, are generally accepted as standard for pediatric MMD.12)13)19) However, there is no study answering the question of which type of surgical technique is practically the most useful in the treatment of adult patients with ischemic type MMD, because there have been no randomized studies to compare the efficacy of surgical techniques, and several retrospective studies have been conducted with various age ranges, small numbers of patients, short-term follow-up, and no standardization of surgical techniques.1)3)4)11)14)15)21)24) In this study, we evaluated the efficacy of single barrel superficial temporal artery (STA)-middle cerebral artery (MCA) bypass for adult patients with ischemic type MMD by reviewing clinical and radiological data.
MATERIALS AND METHODS
Patient population and inclusion criteria
Between 2006 and 2014, a total of 43 single barrel STA-MCA bypass procedures in 43 surgical sessions were performed in 31 adult patients for treatment of ischemic type MMD by two surgeons (S.W.L., J.K.K.). Medical data were collected according to our protocol and reviewed retrospectively under approval of the institutional review board. The inclusion criteria were as follows: 1) patients treated by single barrel STA-MCA bypass surgery, aged ≥ 20 years; 2) diagnosis of definite or probable MMD via digital subtraction angiography (DSA)17); 3) ischemic symptoms including transient ischemic attack (TIA) and cerebral infarction, or old infarction identified by magnetic resonance (MR); 4) preoperative modified Rankin scale score (mRS) ≤ 2; 5) obvious hemodynamic impairment confirmed by single-photon emission computed tomography (SPECT) examinations using acetazolamide (Diamox1) challenge and/or computed tomography perfusion(CTP) preoperatively. Patient's characteristics are shown in Table 1. The male to female ratio was 17:14 and the mean age was 41 years (range, 21-65 years). Among the 31 patients, 28 patients presented with ischemic symptoms and the remaining three patients denied any ischemic symptom. However, ischemic type MMD was diagnosed during evaluation for depression in one patient, and during assessment for traffic accidents in two patients by MR. Four patients presented with TIA preoperatively and probable MMD was diagnosed preoperatively in six patients. Of 43 hemispheres from 31 patients, 21 surgeries were performed on the right hemisphere. Radiologic findings revealed cerebral infarction in 39 hemispheres in the 27 patients.
Surgical technique
All surgically treated patients underwent direct bypass only, without the addition of an indirect revascularization procedure, on the affected side after confirmation of hemodynamic impairment by SPECT and/or CTP. Unless the hemodynamic insufficiency was clearly verified on the opposite side, surgery was done only on the affected side. In the 28 patients presented with ischemic symptoms, bypass surgery was performed between three weeks and three months after a stroke or TIA (mean, 8.3 weeks). Patients who require treatments to both hemispheres are managed with staged operations, with an interval of 8-12 weeks between the two surgeries. Single barrel STA-MCA anastomosis with wide dural opening was performed in all cases. In case of bilateral bypass procedures, surgery was performed on the more symptomatic hemisphere first. Bilateral bypass procedures were performed in twelve patients with one surgical session per side.
Following a linear or curvilinear skin incision of the temporalis muscle, the parietal or frontal branch of the STA was carefully dissected for approximately 10 cm, starting from the external auditory meatus. A large craniotomy was performed more than 10 cm in diameter for wide dural opening. The arachnoid membrane was minimally dissected to expose suitable-sized cortical branches of the MCA as the recipient artery. End-to-side anastomosis of donor and recipient arteries used interrupted 10-0 sutures under an operating microscope. Patency of the anastomosis was confirmed intra-operatively using a hand-held Doppler probe. What kind of an indirect revascularization procedure did not add any more in addition to STA-MCA anastomosis. All patients received 325 mg of acetylsalicylic acid once per day post-operatively. After discharge, dual antiplatelet therapy of clopidogrel and acetylsalicylic acid was maintained for at least three months.
Analysis
For evaluation of clinical outcome, procedure-related complications including postoperative stroke and subsequent ipsilateral stroke beyond 30 days were checked. Postoperative stroke was defined as either infarction or intracerebral hemorrhage, intraventricular hemorrhage, and subarachnoid hemorrhage that developed during the surgery or within 30 days after the surgery. Brain MRI and computed tomography (CT) were performed in cases where the neurological status declined at any time. The preoperative angiographic stage was evaluated according to Suzuki grade.24) Postoperative patency of the bypass was assessed using DSA, MR angiography, or CT angiography. Pre- and postoperative CTP assessment was used to evaluate hemodynamic improvement. Because acetazolamide is not used for CTP in our institute, the cerebrovascular reserve capacity (CVR) was evaluated using SPECT examinations using acetazolamide challenge preoperatively. The CVR was measured as the difference in the regional cerebral blood flow (CBF) after acetazolamide-challenged technetium-99 m-ethyl cysteinate dimer SPECT from baseline regional CBF by visual assessment. All statistical analyses were conducted with the use of statistical software package SPSS (Version 23.0, SPSS, Inc., an IBM Company, Chicago, IL, USA). Because of the skewed distribution of CTP values, a Wilcoxon signed-rank test was used to assess the difference between pre-operative and follow-up within group. The p values of less than 0.05 were considered to indicate statistical significance.
RESULTS
Clinical outcome
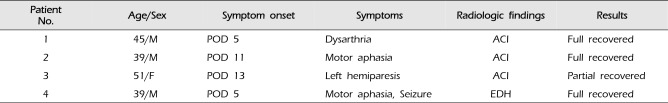
Single barrel STA-MCA anastomosis with wide dural opening was technically successful in all 43 hemispheres. Table 2 summarized procedure-related complications. Permanent complication occurred in only one case of 43 bypass procedures (2.3%). This patient had an infarct in the MCA area 13 days after bypass surgery, after which her neurological status worsened. Further evaluations showed no definite reason for acute infarction. The patient recovered full strength and language function by the time of discharge, although she was left with a residual paresthesia on the left upper limb. Thus, the incidences of permanent neurological morbidity and mortality related to surgery were 2.3% and 0%, respectively. Transient complications were observed in nine cases of 43 bypass procedures (14.0%): six hyperperfusion syndromes, two acute cerebral infarctions, and one acute epidural hematoma at the operating site. Though all patients with these complications were symptomatic initially, they recovered without neurologic symptoms as time goes by. In all six cases with hyperperfusion syndrome, neurological deterioration occurred one day after surgery. MR imaging and CTP and/or SPECT performed at the time showed focal hyperperfusion in the area supplied by the bypass. Symptoms improved within one week after surgery without any deficits. None of the rest of the patients suffered from ipsilateral ischemic events during the 30 days after bypass. Therefore, postoperative stroke was observed in three instances in 43 procedures (6.9%) and the 30-day major adverse events (major stroke and death) rate was 2.3%, respectively.
During the observation period, with a mean of 35 months after surgery (range, 12-73 months), there were no further cerebrovascular events in 29 patients (93.5%). Two patients (6.5%) experienced a subsequent TIA. In these two patients, further evaluation did not reveal the exact cause and the TIA was an isolated event without further ischemic events. In sum, stroke recurrence occurred in two patients (6.5%), resulting in an annual stroke risk of 2.2%.
Angiographic evaluation
Digital subtraction angiography prior to surgery was available to review in all 31 patients. Six patients had probable MMD and underwent revascularization of the affected hemisphere only. Among 25 patients with bilateral MMD, bilateral bypass procedures were performed in 12 patients. Severity of MMD was quantified with Suzuki stage (I-VI, with Stage VI the most severe). The majority of the affected hemispheres (54/56, 96.4%) had Suzuki Stage III-V, and the average Suzuki stage of the study cohort was 4.1.
Follow-up imaging (DSA, MR angiography, or CT angiography) was performed in all 43 revascularized hemispheres to assess patency of the bypass. These studies were performed between two and 59 months after surgery (mean, 16.5 months). Post-operative patency was clearly verified in 38 bypasses (88.4%), while five bypasses (11.6%) were not patent or questionably patent. Post-operative strokes or TIAs did not occurred during the follow-up period in these five patients with occluded bypasses. Although bypasses were not patent or questionably patent in five cases, spontaneous anastomosis, as a result of indirect revascularization, appeared to be helpful in improvement of perfusion in four cases (80.0%).
Hemodynamic evaluation
In all 43 revascularized hemispheres, reduced regional CVR was proven by SPECT examinations using acetazolamide challenge preoperatively. In the current series, SPECT examinations were performed pre/post-operatively to evaluate cerebral hemodynamics and revealed improved cerebral hemodynamics after surgery in most cases. However, this result was excluded from the analysis because of the evaluation method of visual assessment, somewhat less objective. Complete sets of CTP results (pre-operative and follow-up) were obtained for 28 patients with 40 revascularized hemispheres in order to assess changes in CBF, CBV, time to peak (TTP), and mean transit time (MTT). If a patient had two or more CTP studies after bypass surgery, only the last post-operative study was included in this analysis. These radiological exams were performed between six and 42 months after surgery (mean, 18.4 months). The difference of CTP parameters between pre-operative and follow-up is summarized in Table 3. CTP revealed a significant decrease of TTP and MTT, and a significant increase of CBF after surgery (p < 0.001). Representative cases are shown in Fig. 1.

A 48-year old, right-handed male presented with repeated right hemiparesis caused by known MMD. (A) Anteroposterior and lateral views of the left external carotid artery angiogram, obtained preoperatively, demonstrates no abnormal finding. (B) Anteroposterior and lateral views of the left external carotid artery angiogram 12 months after the operation shows that the STA (black arrow)-MCA anastomosis was in good patency and that extensive spontaneous anastomoses of the middle meningeal artery (white arrow) and the deep temporal artery (arrow head) formed with the cerebral cortical arteries. The deep temporal artery, middle meningeal artery, and the trunk of the STA became obviously thickened. (C) CTP scans with TTP and MTT obtained before and after the operation. Significant asymmetry between the right and left hemispheres with striking hypoperfusion of the left ICA territory shows substantial improvement after the operation. MMD = moyamoya disease; STA = superficial temporal artery; MCA = middle cerebral artery; CTP = computed tomography perfusion; TTP = time to peak; MTT = mean transit time.
DISCUSSION
MMD presents with various cerebrovascular events, including TIAs, ischemic stroke, intracranial hemorrhage, headache, or seizures. In adult MMD, approximately half of the patients have intracranial hemorrhage, and the rest of the patients have TIA or cerebral infarct.7)9)23) Although indirect bypass surgery is considered standard treatment in pediatric MMD, there remains some debate about the most effective treatment method in adult MMD.12)13)19) There are several reasons why it is difficult to determine the best treatment method for adult patients with MMD. For instance, the relatively small number of patients and the difficulty of long-term follow-up has prevented establishment of specific surgical techniques.1)3)4)11)14)15)21)24)
In previous reports, it is well recognized that surgical revascularization using direct and/or indirect bypass provides an improved outcome in patients presenting with the ischemic type,3)8) while there are limited reports on the surgical outcome in patients with hemorrhagic MMD.18) However, there is no study answering the question of which type of surgical technique is practically the most useful in the treatment of ischemic type MMD.
Recently, the use of combined direct and indirect [encephaloduroarteriosynangiosis or encephalomyosynangiosis] bypass has been advocated as the optimal treatment in adult MMD.2)10)11)24) According to Cho et al.,2) the annual risks of symptomatic hemorrhage and infarction were 0.4% and 0.2%, respectively, in their 77 operated hemispheres of 60 adult MMD patients after combined revascularization surgery. They concluded that combined revascularization surgery resulted in satisfactory long-term improvement in clinical, angiographic, and hemodynamic states and prevention of recurrent stroke. They speculated the following. Direct revascularization could play its role at the early phase after surgery; thereafter, direct revascularization may maintain its dominant role for collateralization, or as parts of the distal cerebral vessels progressively become occluded (compartmentation), some direct revascularization may slowly regress partly or on the whole. Then indirect revascularization would replace the areas in which blood flow could not reach via direct bypass. In that sense, combined surgery is considered to be the most effective technique. Xu et al.,24) in a study evaluating 111 MMD patients underwent STA-MCA bypass combined with encephaloduromyoarteriosynangiosis, reported this combined revascularization procedure could achieve good therapeutic effect in the treatment of MMD. Although combined direct and indirect anastomosis in patients with MMD immediately increases CBF and decrease the risk of ischemic attack, the surgical procedure is more complex and requires time consuming steps.11) Therefore, this combined revascularization procedure more often may lead to postoperative neurological morbidity, which developed frequently after surgery in brain with hemodynamic compromises and bleeding-prone vasculopathy like MMD. Furthermore, recent studies have shown that postoperative hyperperfusion develops frequently after surgery.6)22) Various types of surgery have been tried since surgical treatment for the MMD began in our institution, and large number of postoperative neurological morbidity have been experienced in the early period of treatment for MMD. The indirect procedure alone potentially decreases CBF in the acute period after the surgery.5)11)20) In the combined revascularization procedure, the immediate increase in CBF from direct bypass probably compensates for the detrimental effect induced by such as indirect procedure, but not perfect. Our observations suggest that various way of indirect revascularization procedures were added to STA-MCA anastomosis could cause or facilitate neurological deficit in the early postoperative period. And we believe that the indirect anastomosis could be established over time according to the demand of CBF, even if an indirect revascularization procedure is not added to STA-MCA anastomosis in MMD patients. In our study, spontaneous anastomosis, as a result of indirect revascularization, appeared to be helpful in improvement of perfusion in four (80.0%) of five patients with non-functioning bypass.
After the trial and error to reduce postoperative morbidity, since January 2006, we have continued to perform single barrel STA-MCA bypass with wide dural opening, without the addition of an indirect revascularization procedure (wide dissecting of arachnoid membrane, inverting dura matter, suturing temporalis muscle to the dura, and the placement of the pericranial flap), as primary treatment for adult MMD. In present series, postoperative stroke was observed in three instances in 43 procedures (6.9%). A recent randomized trial (Carotid Occlusion Surgery Study) reported very high 30-day rates for ipsilateral ischemic stroke (14.4%) after STA-MCA bypass in patients with symptomatic atherosclerotic internal carotid artery occlusion.16) On the other hand, we had a lower stroke rate (6.9%, three cases in 43 procedures) during the same period, with just one permanent deficit, despite including only MMD in our study. Reduction of peri-operative complications may be achieved by a good bypass technique, quality control of anesthesia, blood pressure control, and medical and nursing care during and after the operation. Recently, Kazumata et al.11) reported that the postoperative stroke rate was 7.91% after double barrel STA-MCA bypass combined with EDMS in their 108 adult patients with MMD. These data compare favorably with our results of direct bypass only. However, our result might have a lower risk of postoperative stroke because the incidences of permanent neurological morbidity and mortality related to surgery were 2.3% and 0%, respectively.
The consistent surgical method of single barrel STA-MCA direct bypass only, without the addition of an indirect revascularization procedure, used in this study allowed us to demonstrate the effectiveness of surgical treatment in adult patients with ischemic-type MMD. In this study, we performed 43 surgeries in 31 adult MMD patients with hemodynamic impairment. There were two delayed TIAs (6.5%), and 93.5% (29/31) had no further ischemic or hemorrhagic events after a mean follow up period of 35 months, resulting in an annual risk of 2.2%. No deaths by stroke occurred in this series. Considering the fact that these two TIAs were an isolated event, all patients had a favorable outcome beyond one month after surgery. These midterm outcome in present study compare favorably with other reported series of combined revascularization surgery for adult patients with MMD, although the results should be interpreted with caution because the current study has the major flaws of its retrospective feature and the lack of a control group.10)11)24)
The current study has several limitations. First, this study examined a small number of patients. Second, it was a retrospective observational study. There may have been bias in patient selection and demographics. Third, the follow-up period was not sufficient. MMD is a gradually progressive lesion, and therefore much longer-term follow-up is necessary to investigate the progress of MMD after revascularization. Nonetheless, our findings suggest that single barrel STA-MCA bypass with wide dural opening appears to be a effective treatment strategy in adult patients with ischemic type MMD, given suitable levels of institutional and operator expertise, although these findings should be confirmed by a meta-analysis using large-scale retrospective studies that include a control group.
CONCLUSION
Our results suggest that single barrel STA-MCA bypass with wide dural opening is safe and durable method of cerebral revascularization in adult patients with ischemic type MMD and can be considered as a potential treatment option for adult patients with ischemic type MMD. Further studies with larger patient series and a longer follow up period will be helpful in elucidation of both the efficacy and the longevity of this treatment.
Notes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.