|
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 17(4); 2015 > Article |
|
Abstract
Objective
This retrospective study presents our experience with respect to the clinical and angiographic outcomes of patients treated with stent-assisted coil embolization using Solitaire™ AB stents.
Materials and Methods
From March 2011 to December 2014, 50 patients with 55 wide-necked and/or complex intracranial aneurysms were evaluated. Four patients presented with an acute subarachnoid hemorrhage. Stent deployment was performed with a standard coiling procedure in 49 aneurysms. Three patients underwent bailout stenting, 2 patients were treated by temporary stenting and one patient was treated only by stenting without coiling for dissecting aneurysm.
Results
Successful placement of the Solitaire AB stent was achieved in all the cases. Based on the postprocedural angiographic results, a Raymond 1 was obtained in 32 (59%) of 54 aneurysms, excluded by one case of dissecting aneurysm, and a Raymond 2 in 13 (24%), and a Raymond 3 in 9 (17%). There was one thromboembolic (2%) and three hemorrhagic complications (6%). However, procedure-related morbidity or mortality was not found. Annual follow-up angiographic results from the embolization were obtained in 40 (74.1%) of 54 cases. These results were represented as Raymond 1 in 27 (67.5%), class 2 in 9 (22.5%), and class 3 in 4 (10%) cases. Angiographic improvement associated with progressive thrombosis of the aneurysm was obtained in 10 aneurysms. Four aneurysms were recanalized without requiring additional treatment. In-stent stenosis was found in one aneurysm, but stent migration was not seen on follow-up angiography.
Conclusion
Stent-assisted coil embolization using the Solitaire AB stent for treating wide-necked and/or complex intracranial aneurysms was found to be safe and effective immediately post-embolization and after follow-up. Long-term follow-up will be required to identify the effect of the Solitaire AB stent on recanalization rates.
Endovascular management of wide-necked and/or complex intracranial aneurysms has been a therapeutic challenge with a high risk of recanalization, regrowth, and rerupture.5)9)11)48) Previously, several endovascular strategies for treating these lesions have been performed, such as the balloon remodeling method and the use of 3D coils.1)8)32) The development of endovascular remodeling devices or intracranial stents has made more useful methods available for the treatment of these challenged aneurysms. The use of intracranial stents is helpful in achieving dense packing of coils and in reducing recurrence rates.7)50) Stents also provide support as mechanical scaffolds, preventing coil protrusion into parent vessels.7)50) Currently, several stents are used for stent-assisted coil embolization, including the Enterprise stent (Codman, Raynham, MA, USA), the Neuroform stent (Boston Scientific, Fremont, CA, USA), and the Solitaire™ AB Neuovascular Remodeling Device (ev3, Irvine, CA, USA).
Here we present our experience with stent-assisted coil embolization of wide-necked and/or complex intracranial aneurysms. We evaluated the efficiency of and morbidity associated with the Solitaire AB stent and assessed the impact of this technique on angiographic outcomes.
We investigated retrospectively medical records of patients treated with the Solitaire™ AB Neurovascular Remodeling Device from March 2011 to December 2014. Fifty patients with 55 wide-necked and/or complex cerebral aneurysms received a stent-assisted coil embolization. There were 36 women and 14 men, presenting with a mean age of 51.84 years (range, 32-75 years). These patients were consecutively enrolled. Four patients (8%) have subarachnoid hemorrhage (SAH). Among enrolled patients, the Hunt and Hess (H&H) scale was grade II in two patients (4%) and grade IV and V in one patient (4%). Unruptured intracranial aneurysms were present in 46 patients (92%).
Diagnostic findings were firstly obtained from computed tomographic angiography or magnetic resonance angiography. Before performing all procedures, patients underwent conventional angiography to evaluate both internal carotid arteries (ICA) and vertebral arteries (VA), along with a three-dimensional rotational angiography. The locations of the aneurysms were described in Fig. 1 as follows; paraclinoid of ICA in 35 aneurysms, posterior communicating artery (PcoA) in 8 aneurysms, anterior communicating artery (AcoA) in 3 aneurysms, ophthalmic artery in 2 aneurysms, dorsal wall of ICA in 2 aneurysms, ICA terminus in 1 aneurysm, distal anterior cerebral artery (ACA) in 1 aneurysm, posterior inferior cerebellar artery (PICA) in 1 aneurysm, BA in 1 aneurysm, and posterior cerebral artery (PCA) in 1 aneurysm. Almost 95% of the aneurysms were located in the anterior circulation (n = 52), with the remaining 5% (n = 3) in the posterior circulation. Aneurysm size and morphology were measured using digital subtraction angiography with three-dimensional rotational imaging. Wide-necked aneurysms were defined by a neck width of ≥ 4 mm or a dome-to-neck ratio of < 2. Almost all cases had wide-necked aneurysms, except one with dissection without aneurysmal dilation. Fifty one aneurysms were small (< 10 mm) and 3 aneurysms were large (≥ 10 mm, 25 mm ≤). No giant (> 25 mm) were observed.
In 49 patients, endovascular management was performed through a serial processes as stent was delivered across the aneurysm and deployed to cover its neck and then coils were advanced into the aneurysm sac. A single stenting was performed in one case with dissecting aneurysm. Dual antiplatelet therapy consisted of 100 mg aspirin and 75 mg clopidogrel was administered to patient without SAH every day from 7 days ago before starting procedure. Endovascular procedures were performed under general anesthesia and systemic heparinization. 3,000 IU heparin were administered to patients for achieving an activated clotting time (ACT) between 250 s and 300 s in a bolus intravenously, and 1,000 IU heparin were injected to maintain ACT for each hour thereafter. A unilateral femoral 6-F or 7-F sheath was placed through a percutaneous femoral artery puncture and one 6-F or 7-F guide catheter was inserted into the parent vessel. A Rebar microcatheter was then advanced over a 0.014" microguide wire into the parent artery 1-2 cm beyond the aneurysm. The microcatheter for coiling was navigated into the aneurysmal lumen in order to jail it through deploying the stent. The stent was navigated distally past the aneurysm neck and deployed in the proper position so that each of at least 4 mm from the distal and proximal free margins around the neck to obtain optimal stability. In cases where insufficient alignment occurred, it was technically easy to retrieve and reposition, if necessary, even remove the device before it was detached from the push wire. After achieving the best ideal deployment, the stent was detached from the push wire in all, except two cases with performing temporary stenting. To the next, coiling was performed using bare-platinum coils (Axium, Microplex, Target). In one patient with protruding coil loops of the frame, it was technically feasible to deploy a Solitaire AB stent to push the coil loops into the sac, using the so called "stent-jack technique".18) In three cases with coil loop protrusions or coil mass herniations into the parent vessel caused by stretching, bailout-stenting was necessary. After stent-assisted coil embolization, patients were transferred to the neurologic intensive care unit for careful monitoring vital sign and neurologic status. All patients were heparinized immediately after procedure for 1 day and maintained on dual antiplatelet agents, 75 mg clopidogrel and 100 mg aspirin daily from the next day.
Results of stent-assisted coil embolization were evaluated by conventional cerebral angiography at the end of the procedure to check the degree of aneurysm obliteration. Angiographic results were classified on the basis of Raymond scale depending on the degree of aneurysm occlusion.41) Raymond 1 was defined as complete occlusion of showing no contrast filling at the aneurysmal sac. Raymond 2 was neck remnant of showing residual filling of contrast at the aneurysmal neck, and Raymond 3 was incomplete occlusion of showing residual filling of contrast into the aneurysmal sac due to aneurysmal regrowth or recanalization. Clinical outcomes were investigated immediately post-procedure, at discharge, and at the time of follow-up using the modified Rankin Scale (mRS). Follow-up conventional cerebral angiography and clinical examinations were assessed annually after intervention.
Stent deployment and selective embolization were successfully achieved in all the aneurysms. Upon completion of the intervention, stable stent placement with complete coverage across the aneurysm neck without interrupting parent artery patency was achieved in all patients. Fifty-one of 54 aneurysms were embolized successfully by the jailing or the stent-jack techniques. In 4 aneurysms of them, there was a kick-back of the microcatheter after coil deployment into the aneurysmal sac. The stent was captured and the microcatheter was navigated into the aneurysm. The stent was then deployed again and more coils filled the sac for complete occlusion. Stent retrieval was required in two patients and we performed bailout stenting in three patients. Due to anatomic reasons, in a 53-year-old female patient with a small PICA aneurysm, coiling was impossible after application of the device. She was treated by sole stenting.
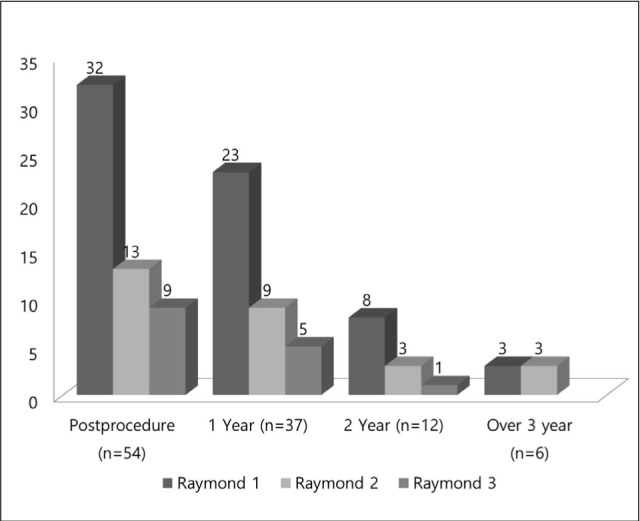
Immediate post-procedural angiographic results were graded as complete occlusion (Raymond 1) in 59% (n = 32) of the 54 aneurysms, neck remnant (Raymond 2) in 24% (n = 13), and residual aneurysm (Raymond 3) in the remaining 17% (n = 9) (Fig. 2). Single stenting for dissecting aneurysm was excluded. Patient characteristics and angiographic results are detailed in Table 1.
One thromboembolic complication (2%) was encountered after Solitaire AB stent deployment when the aneurysmal sac was filled with coils. Local intra-arterial administration of abciximab completely resolved the thrombus even though clinical deficits. Three patients (6%) experienced a temporary neurologic deficit without a restriction on the diffusion-weighted image and completely recovered after heparinization. Three postoperative hemorrhagic complications (6%), which were not located at the aneurysmal locations, developed. These included an intracerebral hemorrhage (n = 1), an intraventricular hemorrhage only (n = 1), and a SAH at the prepontine cistern (n = 1). No arterial dissections, ruptures, or spasms were noted during stent placement in our series.
One (initial H&H grade V) of 4 patients treated in the acute stage of SAH died for reasons unrelated to the endovascular treatment. Two patients left the hospital with a good clinical status; 1 patient with SAH (H&H grade IV) was severely disabled (mRS score 0, n = 1; mRS score 3, n = 1; mRS score 5, n = 1).
Clinical and angiographic follow-up examinations 1 year after procedures were performed for 35 patients with 37 aneurysms (69%, of 54). The angiographic results 1 year after the procedures were graded as Raymond 1 in 62% (n = 23) of the 37 aneurysms, Raymond 2 in 24% (n = 9), and Raymond 3 in the remaining 14% (n = 5) (Fig. 2). Two year follow-up angiographic examinations were performed on 12 aneurysms and on 6 aneurysms at 3 years of follow-up. Follow-up results are presented in Fig. 2.
We analyzed the results of occlusion for aneurysms after treatment based on angiography performed during the last follow-up in all patients. Thirteen aneurysms were lost to follow-up and we are waiting for angiographic follow-up for one aneurysm. Twenty-six (65%) of 40 aneurysms with follow-up data were unchanged (complete occlusion in 20, residual neck in 4 and incomplete occlusion in 2). Ten aneurysms (25%) improved during the follow-up period. Four aneurysms with residual neck and three with incomplete occlusion were completely occluded. Three aneurysms with incomplete occlusion turned into aneurysms with residual neck. However, four aneurysms (10%) were recanalized, although they didn't necessary to treat again anymore. As a consequence, 67.5% of the aneurysms (n = 27) were found to be completely occluded (Raymond grade 1), 22.5% (n = 9) appeared as residual neck (Raymond grade 2), and 10% (n = 4) as incomplete occlusion (Raymond grade 3) after the initial treatment. Clinically asymptomatic in-stent stenosis was observed in the follow-up period in only one patient. Stent migration wasn't seen on follow-up angiography.
A 60-year-old man presenting with headache was found to have dual aneurysms located at the AcoA and the right callosomarginal artery (CMA). We performed stent-assisted coil embolization for the two aneurysms one by one in single procedure. First, we performed stent-assisted coil embolization using the jailing technique for the aneurysm of the CMA. This procedure was successful, resulting in complete occlusion of the aneurysm and procedure- or stent-related complications didn't occur (Fig. 3A). Subsequently, during coil embolization for the aneurysm at the AcoA, the coil loops protruded to the AcoA and we deployed the Solitaire AB stent from the contralateral A2 to ipsilateral A1 across the AcoA. The flow toward contralateral A2 slowed down and became stagnant. We confirmed that an in-stent thrombosis was developed (Fig. 3B) and ipsilateral A1 was occluded completely (Fig. 3C). Due to the certain relation between deployment of the device and the complication, as well to prevent an ischemia, the stent was rapidly retrieved in a technically straight-forward manner without complications and Reopro 2 mg was infused intra-arterially thereafter. Finally, the occluded vessels were completely recanalized (Fig. 3D).
A 35-year-old man with SAH was admitted with H&H grade IV and Fisher grade III. Diagnostic cerebral angiography revealed a 5.4 × 4.4 mm saccular aneurysm originating from the superior and anterior parts of the AcoA (Fig. 4A). Coil embolization was initially performed by using the microcatheter-assisted technique and the two parts of the AcoA aneurysm were occluded completely. However, digital subtraction angiography performed after the detachment of the finishing coil revealed that the AcoA was compromised by protruded coil loops and the contralateral A2 flow slowed down. Also, encroachment of the coil mesh into the ipsilateral A2 was presented (Fig. 4B). We decided to use the self-expandable stent for the bailout procedure due to concerns of parent vessel compromise. The distal parent artery was carefully selected by crossing between the protruded coil mesh and the contralateral parent artery wall. A 4 × 15 mm Solitaire AB stent was navigated and deployed at the site of the protruded coil mesh with successful molding of the protruded coil mesh and preservation of the parent artery (Fig. 4C). Contralateral A2 flow was maintained through the right internal carotid artery (Fig. 4D).
Stent-assisted coil embolization is generally performed using self-expandable stents as a treatment option for large, complex, and/or wide-necked intracranial aneurysms in order to avoid increased periprocedural complications, such as protruded coil loops and to prevent recanalization. The ideal stent for stent-assisted coil embolization should have a low profile, be flexible and self-expandable to accommodate the complex geometry of the intracranial arteries.1) Also, the stent strut design and concentric radial force are important in providing support to prevent the coils from herniating into the parent artery.1)
The Neuroform stent (Boston Scientific, Fremont, CA, USA) was developed as the first self-expandable intracranial stent. This stent has confirmed its superior efficacy and safety in treating wide-necked intracranial aneurysms and also reported lower morbidity and mortality rates associated with the procedure.3)14)31)47)51) However, the Neuroform stent has several limitations, such as the inability to reposition it once partially delivered, a low radial force and some deployment difficulties.14)31) To overcome these limitations, the Leo stent was released as retractable stent for the first time and then the Enterprise stent was developed as an advanced retractable stent. The Enterprise stent is easy to navigate and has been used recently.28)30)35) However, these stents have some limitations, such as the need for larger and stiffer delivery catheters and poor visibility, and the development of new devices will be necessary to overcome these disadvantages.28)30)
Recently, Solitaire™ AB Neurovascular Remodeling Devices has been often used to treat wide-necked and/or complex intracranial aneurysms. They have an excellent navigability as they are delivered through a microcatheter. They are also able to be repositioned even if full deployed because it is possible to be fully retrievable. Therefore, the Solitaire AB stent can be more precisely placed and deployed compared to other stents. This advantage can improve safety and confidence of beginners or trainees in performing stent-assisted coil embolizations. In our cases, we didn't encounter any difficulty or failure in navigation and stent deployment, despite the very tortuous and atherosclerotic vessels. Consequently, we achieved optimal stent positioning in all cases. Excellent flexibility and an easy delivery system through Rebar 0.018 or 0.027 inch microcatheters are needed in order to maximize the high navigability of stents.22)25)
Stenting strategies for stent-assisted coil embolizations have evolved together with the development of new stent devices. These strategies have evolved from the early "microcatether transposition stent technique" and "stent stable microcatheter technique (Jailing)" to newly developed variable techniques, including semi-jailing, simple or multiple stenting for flow diversion, stent-jack, post-releasing, Y-stenting, waffle-cone stenting, and bailout or coil-jailing stenting techniques.1)2)10)12)18)19)20)24)26)34)43)44)45) In our study, we mostly used the jailing technique, along with the stent-jack technique as a variation of the jailing technique. A major advantage of the jailing technique is an improvement in the stability against the kick-back and unstable movement of the microcatheter during coiling in intracranial aneurysms. During coil embolization using the jailing technique in several cases in our study, Solitaire AB stents were recovered multiple times after full release before detachment in order to increase the stability of the microcatheter and to achieve a more dense packing of coils into the intracranial aneurysm. The stent-jack technique is also used to treat broad-necked intracranial aneurysms. First, the coil delivery microcatheter was placed into the aneurysm sac and then the stent was navigated into the parent artery without stent deployment.12) The first coil for framing is advanced into the aneurysm lumen, but if coil loops protruded into the parent artery, the stent is then delivered across the aneurysm neck and deployed to go back to protruded coils' original position by pushing coil loops.12)
The Solitaire AB stent provides the advantage being fully retrievable and redeployable as needed during the procedure until optimal positioning is achieved. This feature can be used to retrieve the stent at the end of the procedure. Several reports have described temporary stent-assisted coil embolization in patients with ruptured aneurysm using the Solitaire AB stent. Signorelli et al.43) and Almekhlafi et al.2) reported that retrieving the stent after coil embolization reduces the need for continued antiplatelet therapy and decreases the hemorrhagic risks associated with antiplatelet use. However, stent retrieval is not recommended in situations where a safety margin is not present or is hard to appreciate due to poor visualization of the stent.2)43) Also, stent retrieval may result in coil herniation and arterial occlusion if some coil loops are tangled inside the stent.2)43) We have two cases in which we performed unintended temporary stenting in unruptured aneurysms. In one case, we failed to reselect the microcatheter into the aneurysm sac after microcatheter kick-back during stent-assisted coil embolization using the jailing technique. The Solitaire AB stent was resheathed and not redeployed because of a stable coil mass and no herniated coil loops. In the other case, the Solitaire AB stent was recaptured due to the development of an in-stent thrombosis after stent deployment at A1 during the performance of a stent-assisted coil embolization for an anterior communicating artery aneurysm. We experienced no complications or technical difficulties in removing the stent.
Sometimes, stents have been used as a bailout procedure to reconstruct the aneurysm sac and/or to restore blood flow of the parent artery during the procedure. Bailout stent deployment with self-expandable stent can be an effective and useful method for reducing or preventing of parent artery compromise or coil migration caused by herniated or unstable coil loops during coil embolization of aneurysms.50) In general, in the final step of coil embolization, forced advancement of the coil into the aneurysm sac may induce stretching, breaking, herniation of coil loops, and coil migration into the parent artery due to be increased the friction and resistance associated with the limited space within the aneurysm sac while the coil is advanced.13)29)40)42) If the coil is advanced compulsively to enter into the aneurysm and temporarily loss the supported force by the attached pusher, the coil may be escaped to the parent artery after detachment due to the sudden loss of force supported by the pusher.29) These conditions may result in thromboembolic complications associated with the prolapsed coils during the embolization of the intracranial aneurysm. Stents have been selected to prevent coil-related thromboembolic complications, with a proximal stent fixation of stretched, herniated, or migrated coils between the wall of the parent artery and the stent. In our series, we performed three bailout stentings due to herniated coil masses or coil loops into the parent artery with compromising parent artery blood flow. These stentings were successfully recovered without any other complications. In these situations, a gentle advancement of the microguide wire and stent passing through the coil loops or mass should be performed to avoid further migration of the herniated coil loops or mass into the distal parent artery or its intracranial branches.
Immediate postprocedural occlusion rates after stent-assisted coil embolization with the Solitaire AB stent were recently reported in a case series of King et al.21) The authors reported complete occlusion in 42.2% of cases and neck remnant in 39.1%. Additionally, Clajus et al.7) reported that the complete occlusion rate was 51%, while neck remnants occurred in 44% of cases. In our series, we obtained a complete occlusion rate of 59% and a neck remnant rate in 24%. Our outcomes were similar to those previously reported for immediate postprocedural occlusions using a Solitaire AB stent.
Stent-assisted coil embolization plays three major roles. First, the stent play a role in supporting the aneurysm neck to fix the coil in the aneurysm sac without protruding inside, and also allows for improved packing density.4) Second, after the deployment of the stent, blood flow in the aneurysm sac is significantly slowed down, which may lead to spontaneous and delayed aneurysm thrombosis, reducing coil compaction in the region of the inflow zone and decreasing the shear stress to the aneurysm wall, while also modifying the hemodynamic characteristics of the parent artery.6)10)17)27)33)49) In our study, the angiographic findings of anatomical improvements with progressive aneurysm thrombosis were revealed in 10 aneurysms (25%) at follow-up periods from 11 to 24 months. Finally, after placement of the stent and filling up with the coils, the stent mesh is embedded into the aneurysm wall to stimulate synthesis of fibroblasts within the arterial wall, which secrete collagen fibers to cover the stent. Meanwhile, the thrombi in the aneurysm and the stent and coil thread at the opening become the scaffolding of collagen fibers. As the collagen fibers gradually grow toward the opening and climb to cover it along the mesh and coil, the latter two in turn facilitate the growth, resulting in the eventual closure of the aneurysm.10)
The major complications related with stent-assisted coil embolization of an aneurysm are thromboembolic or hemorrhagic events, which may result from poor technique, endovascular devices, and/or poor flushing of the catheter systems. We encountered one intraprocedural in-stent thrombosis, three temporary neurologic deficits without restriction of the diffusion-weighted image, and three hemorrhagic complications. These complications may occur in 2.5-28% of patients treated.38)39)46) Increased thrombogenecity has been suggested to result from possible occlusion of parent vessel or distal emboli, and the jailing of perforators after implantation of stents. Appropriate pre- and postprocedural antiplatelet therapy with intravenous heparinization can be safe and can prevent thromboembolic complications. However, hemorrhagic complications can lead to a disastrous outcome owing to periprocedural perforation of an aneurysm, aneurysm rupture, or parent vessel damage if patients take an adequate antithrombotic medication to prevent stent-induced thrombosis and thromboembolic events.23)35)36)37)
Solitaire AB stent induced in-stent stenosis is not well known. This complication is typically late-onset after implantation of stents. The Neuroform stent has been associated with a 5.8% occurrence of delayed moderate or severe in-stent stenosis, 22% of which were symptomatic.15)16) The Enterprise stent had reported in-stent stenosis rates of 8.6-25% in the treatment of aneurysms.31) In our series, although we observed a slight intimal hyperplasia in only one patient on the 1 year follow-up angiogram, there were no clinical events and we did not find it necessary to treat against stenosis. We observed no stent migration because of the stronger radial force of the Solitaire AB stent (0.0106N/mm of stent length) compared to other stents.22)
Solitaire AB stent-assisted coil embolization is a feasible, secure, and effective system with a higher radial force and easy delivery in treating wide-necked and/or complex intracranial aneurysms. The main advantage of the Solitaire AB stent is a complete retrievability even after full deployment. It is therefore possible to locate the optimal position of the stent. The results of our study demonstrated that the Solitaire AB stent is very helpful to achieve high packing densities, complete occlusion, high progressive occlusion, and low recanalization rates. However, we agree that larger studies with long-term follow-ups are required to assess the efficacy and outcomes of Solitaire AB stent-assisted coil embolization in the treatment of wide-necked and/or complex intracranial aneurysms.
References
1. Akpek S, Arat A, Morsi H, Klucznick RP, Strother CM, Mawad ME. Self-expandable stent-assisted coiling of wide-necked intracranial aneurysms: a single-center experience. AJNR Am J Neuroradiol. 2005 5;26(5):1223-1231;


2. Almekhlafi MA, Hockley A, Wong JH, Goyal M. Temporary Solitaire stent neck remodeling in the coiling of ruptured aneurysms. J Neurointerv Surg. 2013 11;5(Suppl 3):iii76-iii78;


3. Benitez RP, Silva MT, Klem J, Veznedaroglu E, Rosenwasser RH. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004 6;54(6):1359-1367; discussion 1368



4. Biondi A, Janardhan V, Katz JM, Salvaggio K, Riina HA, Gobin YP. Neurofrom stent-assisted coil embolization of wide-neck intracranial aneurysms: strategies in stent deployment and midterm follow-up. Neurosurgery. 2007 9;61(3):460-468; discussion 468-9



5. Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999 4;90(4):656-663;


6. Canton G, Levy DI, Lasheras JC. Hemodynamic changes due to stent placement in bifurcating intracranial aneurysms. J Neurosurg. 2005 7;103(1):146-155;


7. Clajus C, Sychra V, Strasilla C, Klisch J. Stent-assisted coil embolization of intracranial aneurysms using the Solitaire™ AB neurovascular remodeling device: initial and midterm follow-up results. Neuroradiology. 2013 5;55(5):629-638;



8. Cloft HJ, Joseph GJ, Tong FC, Goldstein JH, Dion JE. Use of three-dimensional Guglielmi detachable coils in the treatment of wide-necked cerebral aneurysms. AJNR Am J Neuroradiol. 2000 8;21(7):1312-1314;


9. Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999 8;212(2):348-356;


10. Cui YF, Xu H, Liu HT, Wang Y. Clinical application of solitaire AB stents in the embolization of intracranial aneurysms. Eur Rev Med Pharmacol Sci. 2015 4;19(7):1227-1233;

11. Debrun GM, Aletich VA, Kehrli P, Misra M, Ausman JI, Charbel F. Selection of cerebral aneurysms for treatment using Guglielmi detachable coils: the preliminary University of Illinois at Chicago experience. Neurosurgery. 1998 12;43(6):1281-1295; discussion 1296-7


12. de Paula Lucas C, Piotin M, Spelle L, Moret J. Stent-jack technique in stent-assisted coiling of wide-neck aneurysms. Neurosurgery. 2008 5;62(5 Suppl 2):ONS414-ONS416; discussion ONS416-7



13. Fessler RD, Ringer AJ, Qureshi AI, Guterman LR, Hopkins LN. Intracranial stent placement to trap an extruded coil during endovascular aneurysm treatment: technical note. Neurosurgery. 2000 1;46(1):248-251; discussion 251-3



14. Fiorella D, Albuquerque FC, Han P, MacDougrall C. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004 1;54(1):6-16; discussion 16-7



15. Fiorella D, Albuquerque FC, Woo H, Rasmussen PA, Masaryk TJ, McDougall CG. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006 7;59(1):34-42; discussion 34-42


16. Gory B, Klisch J, Bonafé A, Mounayer C, Beaujeux R, Moret J, et al. Solitaire AB stent-assisted coiling of wide-necked intracranial aneurysms: mid-term results from the SOLARE study. Neurosurgery. 2014 9;75(3):215-219; discussion 219



17. Gu DQ, Zhang X, Luo B, Long XA, Duan CZ. The effect of Neuroform stent-assisted coil embolization of wide-necked intracranial aneurysms and clinical factors on progressive aneurysm occlusion on angiographic follow-up. J Clin Neurosci. 2013 2;20(2):244-247;


18. Hong B, Patel NV, Gounis MJ, DeLeo MJ 3rd, Linfante I, Wojak JC, et al. Semi-jailing technique for coil embolization of complex, wide-necked intracranial aneurysms. Neurosurgery. 2009 12;65(6):1131-1138; discussion 1138-9



19. Huang QH, Liu JM, Yang PF, Hong B, Xu Y. Endovascular treatment of intracranial aneurysm using stenting after coiling technique. Chin J Cerebrovasc Disc (Electronic Edition). 2009 3:208-213.
20. Kim YW, Park IS, Baik MW, Jo KW. Endovascular treatment of blood blister-like aneurysms using multiple self-expanding stents. J Korean Neurosurg Soc. 2011 2;49(2):116-119;




21. King B, Vaziri S, Singla A, Fargen KM, Mocco J. Clinical and angiographic outcomes after stent-assisted coiling of cerebral aneurysms with Enterprise and Neuroform stents: a comparative analysis of the literature. J Neurointerv Surg. 2015 12;7(12):905-909;


22. Klisch J, Clajus C, Synchra V, Eger C, Strasilla C, Rosahl S, et al. Coil embolization of anterior circulation aneurysms supported by the Solitaire AB neurovascular remodeling device. Neuroradiology. 2010 5;52(5):349-359;



23. Klisch J, Eger C, Sychra V, Strasilla C, Basche S, Weber J. Stent-assisted coil embolization of posterior circulation aneurysms using Solitaire AB: preliminary experience. Neurosurgery. 2009 8;65(2):258-266; discussion 266



24. Lee JI, Ko JK, Lee TH, Choi CH, Lee SW, Cho WH. Sole stenting technique for the treatment of uncoilable very small aneurysms in the intracranial internal carotid artery. Neurol Med Chir (Tokyo). 2013 53(5):310-317;


25. Lee SY, Chae KS, Rho SJ, Choi HK, Park HS, Ghang CG. Clinical and angiographic outcomes of wide-necked aneurysms treated with the Solitaire AB stent. J Cerebrovasc Endovasc Neurosurg. 2013 9;15(3):158-163;



26. Li CH, Su XH, Zhang B, Han YF, Zhang EW, Yang L, et al. The stent-assisted coil-jailing technique facilitates efficient embolization of tiny cerebral aneurysms. Korean J Radiol. 2014 Nov-Dec;15(6):850-857;



27. Lieber BB, Gounis MJ. The physics of endoluminal stenting in the treatment of cerebrovascular aneurysms. Neurol Res. 2002 24(Suppl 1):S33-S42;


28. Lubicz B, Fran . 231;ois O, Levivier M, Brotchi J, Balériaux D. Preliminary experience with the Enterprise stent for endovascular treatment of complex intracranial aneurysms: Potential advantages and limiting characteristics. Neurosurgery. 2008 5;62(5):1063-1069; discussion 1069-70


29. Luo CB, Chang FC, Teng MM, Guo WY, Chang CY. Stent management of coil herniation in embolization of internal carotid aneurysms. AJNR Am J Neuroradiol. 2008 11;29(10):1951-1955;



30. Lv X, Li Y, Xinjian Y, Jiang C, Wu Z. Results of endovascular treatment for intracranial wide-necked saccular and dissecting aneurysms using the enterprise stent: A single center experience. Eur J Radiol. 2012 6;81(6):1179-1183;


31. Lylyk P, Ferrario A, Pasbon B, Miranda C, Doroszuk G. Buenos Aires experience with the Neuroform self-expanding stent for the treatment of intracranial aneurysms. J Neurosurg. 2005 2;102(2):235-241;


32. Malek AM, Higashida RT, Phatouros CC, Dowd CF, Halbach VV. Treatment of an intracranial aneurysm using a new three-dimensional-shape Guglielmi detachable coil: technical case report. Neurosurgery. 1999 5;44(5):1142-1144; discussion 1144-5



33. Mangiafico S, Guarnieri G, Consoli A, Ambrosanio G, Muto M. Endovascular strategy for unruptured cerebral aneurysms. Eur J Radiol. 2013 10;82(10):1638-1645;


34. Martínez-Gáldamez M, Saura P, Aaura J, Martínez A, De Campos JM, Pérez A. Y-stent-assisted coil embolization of anterior circulation aneurysms using two Solitaire AB devices: a single center experience. Interv Neuroradiol. 2012 6;18(2):158-163;



35. Mocco J, Snyder KV, Albuquerque FC, Bendok BR, Alan SB, Carpenter JS, et al. Treatment of intracranial aneurysms with the Enterprise stent: A multicenter registry. J Neurosurg. 2009 1;110(1):35-39;


36. Moret J, Cognard C, Weill A, Castaings L, Rey A. Reconstruction technique in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol. 1997 6;24(1):30-44;

37. Pandey AS, Koebbe C, Rosenwasser RH, Veznedaroglu E. Endovascular coil embolization of ruptured and unruptured posterior circulation aneurysms: Review of a 10-year experience. Neurosurgery. 2007 4;60(4):626-636; discussion 636-7



38. Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2005 3;26(3):506-514;


39. Pelz DM, Lownie SP, Fox AJ. Thromboembolic events associated with the treatment of cerebral aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol. 1998 9;19(8):1541-1547;


40. Phatouros CC, McConachie NS, Jaspan T. Post-procedure migration of Guglielmi detachable coils and mechanical detachable spirals. Neuroradiology. 1999 5;41(5):324-327;



41. Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997 12;41(6):1235-1245; discussion 1245-6



42. Schutz A, Solymosi L, Vince GH, Bendszus M. Proximal stent fixation of fractured coils: technical note. Neuroradiology. 2005 11;47(11):874-878;



43. Signorelli F, Gory B, Turjman F. Temporary Solitaire stent-assisted coiling: a technique for the treatment of acutely ruptured wide-neck intracranial aneurysms. AJNR Am J Neuroradiol. 2014 5;35(5):984-988;



44. Spiotta AM, Wheeler AM, Smithason S, Hui F, Moskowitz S. Comparison of techniques for stent assisted coil embolization of aneurysms. J Neurointerv Surg. 2012 9;4(5):339-344;


45. Sychra V, Klisch J, Werner M, Dettenborn C, Petrovitch A, Strasilla C, et al. Waffle-cone technique with Solitaire™ AB remodeling device: endovascular treatment of highly selected complex cerebral aneurysms. Neuroradiology. 2011 12;53(12):961-972;



46. Vinuela F, Duckwiler G, Maward M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997 3;86(3):475-482;


47. Wu Z, Lu X, Yang X, He H. Ruptured vertebra-inferioposterior cerebellar artery dissecting aneurysm treated with the Neuroform stent deployment and vertebral artery occlusion. Eur J Radiol Extra. 2009 70:e100-e103.

48. Yavuz K, Geyik S, Pamuk AG, Koc O, Saatci I, Cekirqe HS. Immediate and midterm follow-up results of using an electrodetachable, fully retrievable SOLO stent system in the endovascular coil occlusion of wide-necked cerebral aneurysms. J Neurosurg. 2007 7;107(1):49-55;

49. Ye HW, Liu YQ, Wang QJ, Zheng T, Cui XB, Gao YY, et al. Comparison between Solitaire™ AB and Enterprise stent-assisted coiling for intracranial aneurysms. Exp Ther Med. 2015 7;10(1):145-153;



Fig. 1
Location of aneurysms. ACA = anterior cerebral artery, AcoA = anterior communicating artery, BA = basilar artery, ICA = internal carotid artery, OPH = ophthalmic artery, PCA = posterior cerebral artery, PcoA = posterior communicating artery, PICA = posterior inferior cerebellar artery.
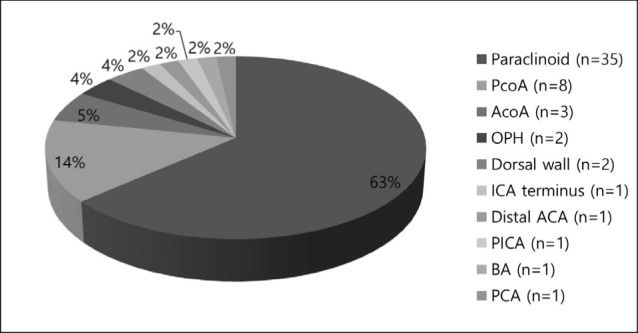
Fig. 3
A 60-year-old man presenting with headache is found to have dual aneurysms located at the anterior communicating artery (AcoA) and the right callosomarginal artery (CMA). (A) An arteriogram of the left internal carotid artery shows a complete occlusion of the right CMA aneurysm after stent-assisted coil embolization. (B, C) Subsequently, coiling for the AcoA aneurysm is started without stenting. During coiling, the coil loops become a coil protrusion into the AcoA. After the placement of the Solitaire AB stent, the aneurysm is completely occluded. However, in-stent thrombosis does develop at the contralateral proximal A2 (black arrow) and finally, the ipsilateral A1 is totally occluded. (D) The stent is removed as soon as possible and the occluded arteries are completely recanalized.
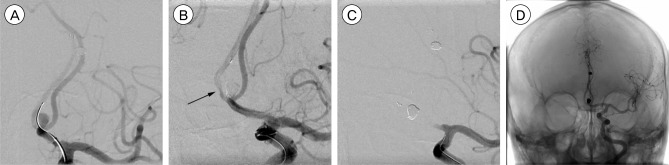
Fig. 4
A 35-year-old man presents with semicomatous mentality and computed tomographic scans reveal a thick subarachnoid hemorrhage. (A) An arteriogram of the left internal carotid artery shows an aneurysm originating from the superior and anterior parts (white arrow) of the anterior communicating artery (AcoA). (B) Coiling is performed and the two parts of the AcoA aneurysm are occluded. However, the AcoA is compromised by protruded coil loops and the contralateral A2 flow slows down (black arrow). Also, encroachment of the coil mesh into the ipsilateral A2 is noted (black arrow heads). (C) Due to concerns of ipsilateral A2 compromise, a Solitaire AB stent is deployed at the site of the protruded coil mesh, achieving successful molding of the protruded coil mesh (white arrow heads). (D) Contralateral A2 flow is maintained through the right internal carotid artery.

Table 1
Summary of patient characteristics and outcome
ACA = anterior cerebral artery; AcoA = anterior communicating artery; BA = basilar artery; ICA = internal carotid artery; ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage; OPH = ophthalmic artery; PCA = posterior cerebral artery; PcoA = Posterior communicating artery; SAH = subarachnoid hemorrhage
- TOOLS
-
METRICS

-
- 9 Crossref
- 0 Scopus
- 2,134 View
- 23 Download
- Related articles
-
Stent-assisted Coil Embolization of Cerebral Aneurysms: Review Article.2011 March;13(1)
Single-session Coil Embolization of Multiple Intracranial Aneurysms2013 September;15(3)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print