Spontaneous Occluded Anterior Communicating Artery Aneurysm during Coil Embolization Treated with One Coil Insertion into Remaining Stump
Article information
Abstract
Spontaneous thrombosis of a ruptured aneurysm during coil embolization is a rare event, and some reports on recanalization of a spontaneous occluded ruptured aneurysm have been published. We report on a case of a 54-year-old male who presented with a subarachnoid hemorrhage due to rupture of a small aneurysm of the anterior communicating artery (ACoA). Cerebral angiography confirmed the presence of the ACoA aneurysm, but, during coil embolization, the aneurysm was near completely occluded with a remaining small neck. A small coil was inserted into the remaining stump of the neck to prevent recanalization, and the angiographic result at 1 year after coil embolization showed complete obliteration of the aneurysm.
INTRODUCTION
Although spontaneous thrombosis of large and giant aneurysms is not uncommon, this situation in a small ruptured cerebral aneurysm is a rare event with incidence of 1-2%. Its possible pathophysiology with contributing factors has been well discussed in the literature.1)2)3)8)12)15)18) In addition, subsequent recanalization within the next few weeks has also been described. We report on a case of acute thrombosis of the aneurysm, which occurred immediately after coil extraction, and suggest the next way to prevent recanalization, which can lead to re-rupture of the aneurysm.
CASE REPORT
History
A 54-year-old male experienced a sudden onset of headache which had developed 2 days ago. After the initial diagnosis of subarachnoid hemorrhage (SAH) using brain computed tomography (CT) in another hospital, he was transferred to the emergency room where his Glasgow Coma Scale score was E4V5M5. Despite neck stiffness, there was no focal neurological sign. His medical history was non-contributory and laboratory blood tests found no abnormalities.
Radiologic findings
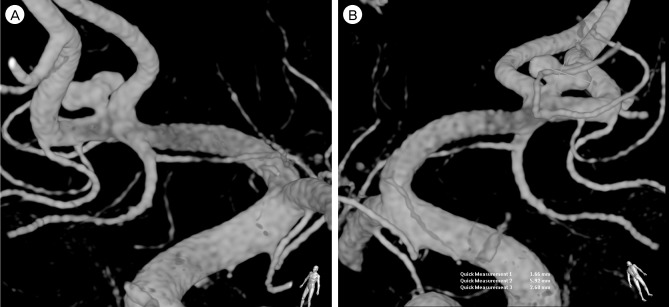
A brain CT scan taken on admission showed focal SAH concentrated in the anterior portion of the basal cistern (Fig. 1). Digital subtraction angiography (DSA) confirmed the presence of the anterior communicating artery (ACoA) aneurysm and 3D rotational angiography (3DRA) showed that the aneurysm had a very narrow neck, which measured 5.9 × 2.7 × 1.7 (width × height × neck diameter) (Fig. 2). Based on the distribution of the SAH, we considered that the ACoA aneurysm had bled. As morphologic features of the aneurysm were adequate for coil embolization, we decided to perform an emergency endovascular coil embolization on the day of admission.
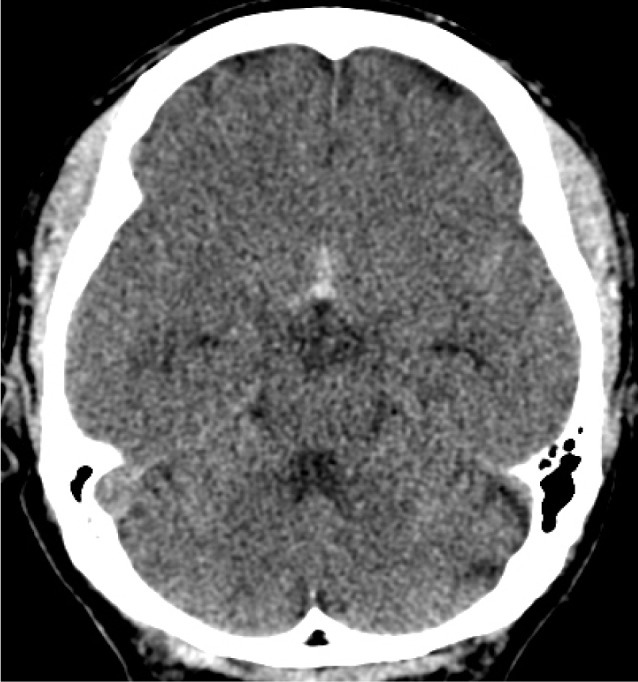
Axial brain CT scan taken on admission shows a subarachnoid hemorrhage on the anterior portion of the basal cistern. CT = computed tomography.
Endovascular treatment
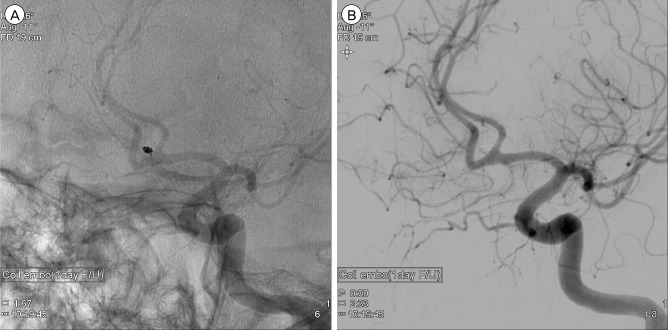
The embolization procedure was performed with the patients under general anesthesia. DSA was performed again to assure the aneurysm. After confirming that the result on DSA was identical to the previous one, a microcatheter was carefully guided over a microguidewire into the aneurysm. As the tip of the catheter was kept at the neck of the aneurysm, the first coil (Target® Detachable coil 3 mm × 8 cm; Stryker Neurovascular, Fremont, CA, USA) placement was attempted very slowly. At the last moment of deploying the first coil, the tip of the microcatheter was seen to move back from the aneurysm and the projection of the coil loop outside the aneurysm was observed (Fig. 3A). Consequently, the first coil was gently withdrawn from the aneurysm. However, a subsequent angiogram showed near obliteration of the aneurysm without evidence of vasospasm. Only a tiny stump at the neck was noted (Fig. 3B). Because acute thrombosis facilitated by an exposed coil within the aneurysm was assumed, and, sooner or later, recanalization could occur, we decided to insert a smaller coil into the remaining stump of the neck. With the coil (HyperSoft® 3D complex Coil 2 mm × 4 cm; MicroVention, Tustin, CA, USA), embolization was performed safely and post-embolization DSA showed complete occlusion of the aneurysm (Fig. 3C, D). There was no occurrence of procedure-related complication during coil embolization and the patient recovered without neurologic deficit after the procedure.

Neurointerventional images during coil embolization. At the moment of deploying the first coil, the tip of the microcatheter is seen to move back from the aneurysm and the projection of the coil loop outside the aneurysm is observed (A). After extraction of the coil, subsequent angiogram shows near obliteration of the aneurysm (B). Insertion of a smaller coil into the remaining stump of the neck is performed (C) and post-embolization angiogram shows complete occlusion of the aneurysm (D).
Follow-up
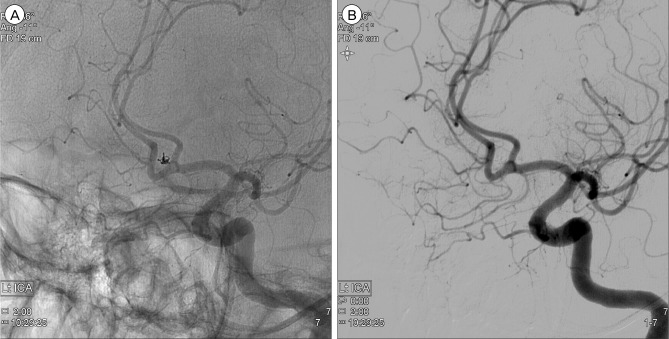
Follow-up DSA the next day showed persistent occlusion of the aneurysm (Fig. 4). The patient was discharged without neurologic deficit of the modified Rankin scale score 0. Magnetic resonance (MR) angiography at 3-month follow-up showed no evidence of residual neck or recurrence of recanalization. On 1 year follow-up DSA, despite change in the configuration of the coil, the aneurysm remained to be completely obliterated (Fig. 5).
DISCUSSION
The reported incidence of acute thrombosis of the aneurysm after SAH is 1-2%,7)9)17) and several possible mechanisms have been reported in the literature. In large or giant aneurysms, in particular, the incidence of spontaneous total thrombosis of the aneurysm has shown a marked increase, ranging from 13% to 20%,10) and the volume-to-orifice ratio of the aneurysm has been suggested as the most reliable mechanism of spontaneous thrombosis.1) However, in small aneurysms, other factors including anti-fibrinolytic agent, non-ionic contrast media, systemic hypotension, increased blood coagulability, increased platelet aggregation, vasospasm, and hemodynamics in the parent artery have also been suggested as an influential mechanism.3)6)8)15)18)20) In our patient, we supposed several factors that promoted thrombosis of the aneurysm. The most important and potent factor was a narrow neck of the aneurysm of 1.7 mm with a large volume-to-orifice ratio. Extremely narrow neck of the aneurysm was regarded as sufficient potential to induce thrombosis under special circumstances like the following. First, the endovascular procedure - including microcatheter placement on the neck of the aneurysm and aborted first coil - could interrupt the intrasaccular blood flow and trigger aneurysm thrombosis. Second, inadequate systemic heparinization might induce spontaneous thrombosis. According to the protocol of our institute, we generally induce systemic heparinization after successful placement of the first basket coil in treatment of a ruptured cerebral aneurysm. In this patient, systemic heparin was not administered during the procedure, and the endovascular procedure was performed under inadequate systemic heparinization. Third, induced hypotension during the procedure could be a potential factor in spontaneous thrombosis. From this experience, if the neck of a ruptured aneurysm was very narrow, a rapid and adequate endovascular procedure, sufficient heparinization and exclusion of excessive hypotension during the procedure should be considered in order to avoid spontaneous thrombosis of the ruptured aneurysm.
Lee et al.13) pointed out that the mechanism of recanalization might be the result of liquefaction of the thrombus and subsequent intrathrombotic dissection by blood flow. Another opinion regarding recanalization following thrombosis of the aneurysm suggests that when the thrombosis was induced by endovascular treatment, recanalization of the thrombosed aneurysm might occur spontaneously.14) Although the pathophysiology of spontaneous recanalization has not been fully elucidated, recanalization ensuing from complete thrombosis of aneurysms has been reported in many studies.4)5)11)12)16)19)21)22) In these studies, recanalization of acute thrombosis of the aneurysm was observed in the days and weeks after thrombosis. Therefore, follow-up angiogram and careful observation during the follow-up period are required for fear of recanalization resulting in rebleeding of the aneurysm. In our patient, coil embolization was performed using a small coil in the tiny stump of the remaining neck. We believed that this procedure might prevent spontaneous recanalization rather than inducing recanalization because the inserted coil would generate an additional thrombus as much as to block the narrow neck, thus resulting in obstruction of blood flow. Fortunately, a satisfactory result was obtained with complete occlusion of the aneurysm after 1-year follow-up.
CONCLUSION
Thrombosis in a ruptured small aneurysm may be generated during coil insertion and the extraction process when the neck of the aneurysm is very narrow. Because recanalization may occur within the next few weeks, we believe that coil insertion on the remaining neck would provide a measure to prevent spontaneous recanalization.
Notes
Disclosure: The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.