INTRODUCTION
Holmes' tremor, first described by Gordon Holmes in 1904, is a rare condition characterized by symptomatic low frequency resting tremor, usually less than 4.5 Hz, which is enhanced by posture, even more aggravated with action, disappears during sleep, and is generally unilateral.2) This tremor, also known as rubral, midbrain, or myorhythmia,6) typically involves the proximal regions of the limbs and occurs as a delayed manifestation of lesions involving the upper brain stem, areas adjacent to the red nucleus, substantia, and the nigrostriatal tract.9) Hypertrophic olivary degeneration (HOD) is a rare form of trans-synaptic neuronal degeneration representing the final result of alteration in the Guillain-Mollaret triangle (denato-rubro-olivary pathway).3)
Holmes' tremor is correlated with HOD.9) Brainstem insults leading to HOD include infarction, infection, tumors, hemorrhage, and other demyelinating diseases. We report on a patient with brainstem hemorrhage whose magnetic resonance imaging (MRI) showed evidence of bilateral HOD, Holmes' tremor, and a palatal myoclonus.
CASE REPORT
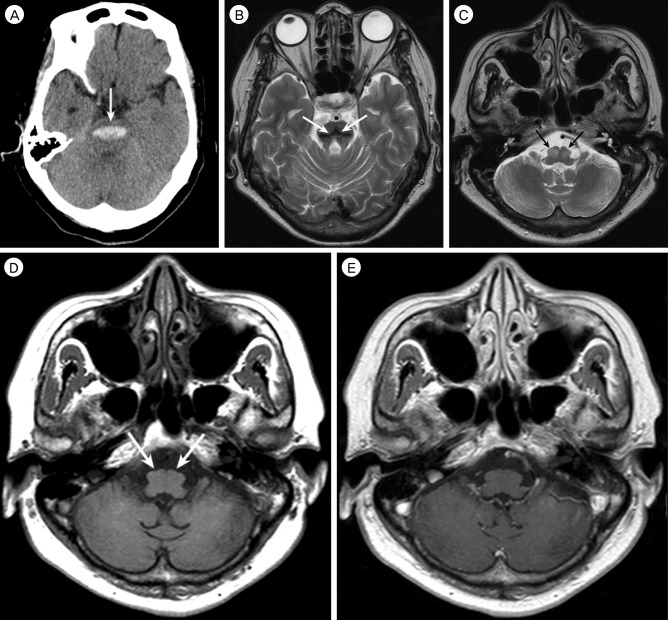
A 59-year-old woman who had hypertension was admitted due to sudden deterioration of a semicomatous mental state. Neurological examination revealed pin point pupil, extension to pain, increased deep tendon reflexes, and positive for other pathologic reflexes. Brain computed tomography (CT) scan on admission showed an acute hemorrhage in the pons (Fig. 1A). The patient received conservative management in the Intensive Care Unit, and was eventually transferred to a rehabilitation facility at one month post-hemorrhage, where she continued to have quadriparesis, facial weakness, dysphasia requiring a feeding tube, and profound disequilibrium. Her condition showed gradual improvement over several months; she became alert, but still had significant diplopia, dysarthria, and paresthesia.
Seven months after the brainstem hemorrhage, the patient was reassessed because of deteriorated dysarthria and diplopia. She had low amplitude, irregular, coarse tremor of the right upper extremity at rest and posture that was further accentuated by action and did not occur during sleep. The tremor was flexion-extension oscillatory movement at the right upper extremity, most predominant distally, with a frequency of 2-4 Hz. The patient also developed a bilateral palatal and irregular dystonic head tremor with aggravated horizontal diplopia. Distinct limitation of upward and downward gaze was observed in both eyes. Ptosis was observed bilaterally. Sitting was difficult without support.
Brain MRI showed residual hemosiderin in the area of the hemorrhage (Fig. 1B) and bilateral symmetrical hypertrophy in the bilateral inferior olivary nuclei. These lesions were hyperintense on a T2-weighted image and isointense on a T1-weighted image (Figs. 1C and D). A gadolinium enhanced T1-weighted image did not show contrast enhancement (Fig. 1E). Diagnosis of bilateral HOD was made.
Her neurologic function worsened over the subsequent days. As these symptoms progressed, her ability to perform activities of daily living worsened, including her ability to eat independently. She was treated sequentially with propranolol, clonazepam, trihexyphenidyl, and levitracetam, but her symptoms persisted. Her medication was switched to gabapentin (900 mg) for her sensory complaints and levodopa/carbidopa (250/25 mg) for the tremor. Initially, she was treated with small doses of levodopa/carbidopa but the dosage was increased progressively over the course of three months to reach a final dose of 250/25 mg three times per day. Despite treatment with these drugs for three months, her tremor showed little improvement.
DISCUSSION
Brain stem hemorrhage can cause development of Holmes' tremor.11) The Movement Disorder Society has established three criteria for the diagnosis of Holmes' tremor; (1) presence of both resting and intentional tremor, (2) a slow frequency, usually less than 4.5 Hz, and (3) a variable delay (usually months) after occurrence of the lesion.2) Holmes' tremor is also known as a rubral tremor or midbrain tremor. Although predominantly an action tremor, rubral tremor frequently has a significant resting component. The amplitude of the movement tends to be large and it can sometimes adopt a wing-beating appearance. In our case, the features were consistent with the reported criteria; (1) low amplitude, irregular, coarse tremor at rest and posture, further accentuated by action and not occurring during sleep; (2) predominantly unilateral; (3) the tremor began seven months after the brainstem hemorrhage. As with cerebellar and symptomatic palatal tremor, rubral tremor arises from damage to the cerebellar and brainstem motor pathways and from dysregulation of motor control during movement. The pathogenesis of Holmes' tremor has been attributed to combined destruction of the pallidothalamic and cerebellothalamic pathways, with involvement of the rubro-olivo-cerebello-rubral loops and possibly the nigrostriatal system. This patient developed Holmes' tremor on the right upper extremity and brain MRI showed evidence of HOD seven months after the brain stem hemorrhage; palatal tremor was also observed. There was no torticollis or tremor of the lower limbs. Symptomatic palatal tremor is a pathologically and anatomically well-defined disorder that commonly appears after disruption of the Guillain-Mollaret triangle (also known as the dentato-rubro-olivary pathway).3) Palatal tremor is correlated with HOD. An unusual form of transneuronal degeneration is the enlargement, rather than atrophy, of the affected inferior olivary nuclei.13) HOD is predominantly unilateral; however, rare bilateral cases have been reported.5)7) Midline lesion or lesions in the superior cerebellar peduncle can result in bilateral HOD (interrupting decussation of the dentato-rubro-olivary pathway).1)
Central nervous system degeneration leads to atrophy characterized by neuronal loss and proliferation of glial elements. Pathological findings of HOD are cytoplasmic vacuolar degeneration, neuronal enlargement, gliosis, and glial hypertrophy. These changes lead to macroscopic enlargement of the olive with corresponding non-enhancing focus of T2 hyperintensity on MRI, which is characteristic of HOD. The differential diagnosis for a T2 hyperintense lesion in the olivary region includes infarct, neoplasm, demyelinating process, and infection. However, the lack of enhancement excludes the majority of neoplastic and infectious entities and enlargement of the olive would be atypical in the setting of infarction or chronic demyelination. The most important clue is its association with the inciting lesion of the contralateral cerebellum or ipsilateral brain stem.
Holmes' tremor usually begins weeks to months after brainstem insult. The delayed-onset between insult and tremor following a structural lesion of the brain is well described but poorly understood. The delay between brain stem insult and tremor is unpredictable but usually occurs at around four weeks to two years. This may be related to transneuronal plastic changes occurring at the neuronal level.9)10)
In review of the literature, we found a few case reports of Holmes' tremor secondary to brainstem hemorrhage that responded to levodopa.8)12) However, the drug therapy is usually unsuccessful. Because the success rate of medical treatment is low, some patients are referred for surgery. The commonly chosen target for deep brain stimulation (DBS) is the ventrolateral nucleus of the thalamus. Thalamic DBS has therefore often been applied for the surgical treatment of Holmes' tremor.4) Treatment of Holmes' tremor should be individualized. A major goal is to keep the patient functioning independently as long as possible. Medical therapy alone is less likely to be effective in such cases. Surgery may be considered for patients who do not respond to the usual medical treatment.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print






