Spontaneous Thrombolysis of Multiple Thrombi at Distal Region of Hypoplastic Vertebral Artery After Stent-assisted Angioplasty on Vertebral Artery Origin Stenosis: Angiographic Follow-up
Article information
Abstract
Vertebral artery hypoplasia (VAH) can be easily overlooked if the contralateral side vertebral artery is intact, because of compensation by the contralateral artery or cerebral collateral network. The clinical relevance and hemodynamic impact of VAH is still controversial. However, VAH has recently been considered a risk factor for posterior circulation ischemia. Ischemic stroke is seldom caused by free floating thrombi (FFT) in the artery. Pathophysiology of FFT has not yet been clarified. The state of reduced blood flow such as a vertebral artery origin stenosis may cause FFT. Their instability may make them sources of recurrent artery to artery embolism. Patients with FFT will require appropriate medical and endovascular treatment.
The current case illustrates a short-term angiographic change of spontaneous thrombolysis of VAH and multiple thrombi at the distal region of the stenosed lesion after stent-assisted angioplasty for a vertebral artery origin stenosis.
INTRODUCTION
Congenital anatomical variations in both vertebral arteries are relatively frequent; left vertebral artery dominance presents in 50% of the population, whereas similar sized vertebral arteries present with only 25% prevalence. Due to this high prevalence, vertebral artery hypoplasia (VAH) has not been considered an independent risk factor for ischemic stroke.8) In the early 1990s, a magnetic resonance angiography (MRA) study found that symptomatic or asymptomatic pontine infarctions were twice as frequent in patients with VAH as in symmetric vertebral arteries.19) Emerging evidence suggests that VAH may contribute to posterior circulation ischemic events, particularly when there are other coexisting risk factors.6)9)
Thrombus formation occurs as a result of a hemodynamic imbalance in various factors. Antithrombotic treatment is the primary therapeutic option for prophylaxis and treatment of ischemic stroke. However, antithrombotic treatment is less effective for prevention of thrombus formation in stenotic arteries, which is largely dependent on platelet function to aggregate under high shear conditions in regions of stenosis. Multiple free floating thrombi are occasionally noted in the distal region of stenosed arteries. In such situations, angioplasty may provide effective management for improvement of blood flow and prevention of additional thrombus formation.
We report on a rare case of a short-term angiographic change that resulted in spontaneous thrombolysis of multiple thrombi at a distal region after stent-assisted angioplasty in a patient with acute ischemic stroke due to multiple thrombi complicated by a vertebral artery origin stenosis.
CASE REPORT
A previously healthy 60-year-old male presented with dizziness, mild headache, and a sudden bilateral visual field defect for the past three days. The patient had no specific medical history. The patient had left homonymous hemianopsia without abnormality in ocular movement and no motor weakness in both extremities. Muscle tone, strength, rapid alternating movements, cerebellar and sensory examinations, and stance and gaits were all normal. Laboratory blood tests including coagulation and fibrinolysis profiles were all within normal ranges. Electrocardiogram and a chest radiograph showed no abnormalities. Diffusion-weighted MR imaging (DW-MRI) of the brain showed multiple areas of high signal intensity in the right cerebellar hemisphere, occipital area, and splenium of the corpus callosum (Fig. 1A, B). Both vertebral arteries were noted on intracranial MRA (Fig. 1C). However, gadolinium-enhanced cervical MRA demonstrated a prominent left vertebral artery and an invisible long segment of the proximal right vertebral artery (Fig. 1D). First, the patient was treated conservatively with anti-platelet medications (75 mg clopidogrel and 100 mg aspirin daily). Despite receiving anti-thrombotic treatment, he developed sudden dysarthria and dizziness on day 7 after admission. A repeat DW-MRI after development of symptoms showed no new parenchymal lesions.
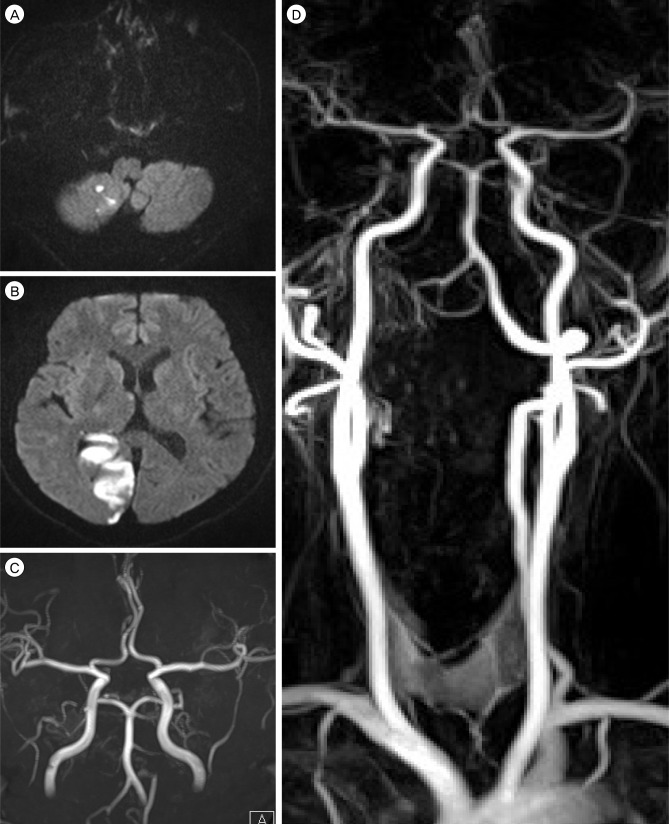
(A,B) Diffusion weighted magnetic resonance imaging (MRI) of the brain shows focal high signal intensities in the right cerebellar hemisphere, occipital area, and splenium of the corpus callosum. (C) No significant steno-occlusive lesions are detected on intracranial MR angiography (MRA). (D) Gadolinium-enhanced cervical MRA demonstrates the prominent left vertebral artery and invisible long segment of the proximal right vertebral artery.
The patient had recurrent ischemic attacks despite receiving anti-platelet medication. We performed transfemoral cerebral angiography. Right common femoral artery access was obtained under local anesthesia. A baseline control angiogram of the right vertebral artery showed severe stenosis of the right vertebral artery origin and multiple filling defects in the region distal to the stenosis (Fig. 2A). The contralateral side of the vertebral artery was intact. The patient was diagnosed with a recurrent ischemic attack due to vertebral artery origin stenosis, therefore, we performed stent-assisted angioplasty.
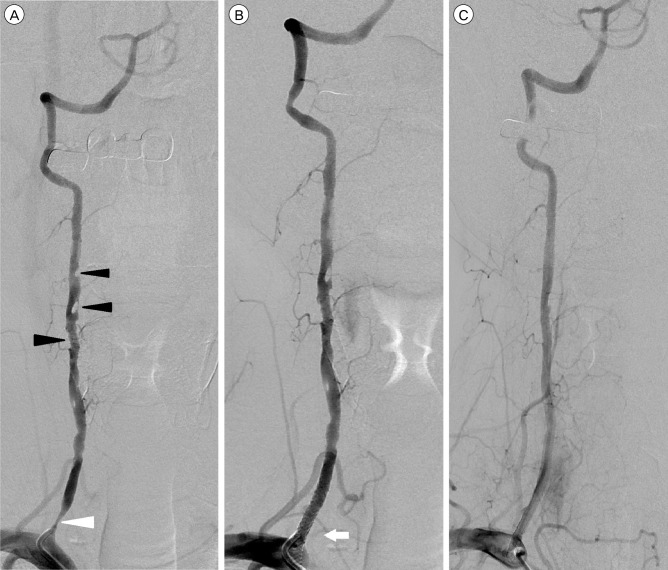
(A) Pre-stenting angiogram of the right vertebral artery shows severe stenosis of the right vertebral artery origin (white arrowhead) and multiple filling defects distal to the stenosis (black arrowheads). (B) Angiogram obtained immediately after vertebral artery origin stent placement shows significant improvement in the stenosis (white arrow) and continued multiple filling defects in the right vertebral artery. (C) A two-week follow-up angiogram shows a patent vertebral artery orifice after placement of a stent and the previously noted multiple thrombi in the right vertebral artery had disappeared.
The right vertebral artery was navigated using a microcatheter and microwire. Then, an embolic protection device (4.0 mm; SpiderFX, ev3, Covidien, Plymouth, MN, United States) was deployed in a straight V2 segment of the right vertebral artery after carefully manipulating it across the stenosis and the thrombus. Subsequently, a balloon-mounted stent (2.75×23 mm; Xience V, Abbott, IL, United States) was successfully deployed, and the embolic protection device was retrieved. There was no thrombus formation or debris within the protection device. An immediate post-stent angiogram showed significant improvement in the stenosis, however, multiple thrombi were observed in the V2 segment of the vertebral artery (Fig. 2B). Due to the risk of further distal emboli or migration, we have decided not to go ahead with additional mechanical or chemical thrombectomy.
The patient continued on 75 mg clopidogrel and 100 mg aspirin daily. Two-week follow-up angiography after the procedure showed no recurrence of the right vertebral artery origin stenosis and the previously noted multiple thrombi in the right vertebral artery had disappeared (Fig. 2C). The lesion was treated successfully with a vertebral artery origin stent. Multiple fragments of the thrombus disappeared spontaneously. Although the patient complained of mild dizziness and left homonymous hemianopsia, there was no further progression of neurological symptoms. Three weeks after admission, he was discharged. At the time of discharge he had no new neurological deficits.
DISCUSSION
VAH was initially described in the 19th century.16) Congenital anatomical variations in both vertebral arteries are relatively frequent, as left vertebral artery dominance presents in 50% of the population. VAH is a congenital vertebral artery abnormality. The sympathetic nervous system induces smooth muscle constriction. The importance of VAH for brain perfusion has been discussed.18) One author reported that VAH can be defined as a vertebral artery < 2.0 mm in diameter by time-of-flight MRA (TOF-MRA).8) Another study found in the literature reported that the diameter of a hypoplastic vertebral artery is 2-2.8 mm by ultrasonography.18) The differential diagnosis between VAH, aplasia, occlusion, and dissection may be difficult. Even conventional angiography and MRA may fail to reliably distinguish VAH or aplasia from vascular occlusion, as they only demonstrate intraluminal flow and cannot visualize the vascular walls.14) Caplan et al. first reported that occlusion is more likely to occur with smaller paired arteries compared with their larger counterparts.5) The underlying cause may be differences of physics of blood flow and shear forces between small and large arteries. A small-diameter artery appears to be more vulnerable to stenosis or occlusion.14) In a sonographic evaluation of pediatric patients with a history of headache, vertigo, or syncope, decreased vertebral artery diameter was associated with both reduced blood flow and inadequate blood supply to the posterior circulation.4) A posterior circulation stroke may occur due to artery-to-artery embolism from a low-flow stenotic vertebral artery.16) The posterior circulation is presumably more vulnerable to ischemia in patients with VAH.14) When a patient with posterior circulation ischemic stroke is VAH on initial contrast enhanced MRA, it is suspicious that VAH contributed to ischemic stroke.
Thrombosis is a pathological consequence of the normal hemostatic process. Under pathological conditions such as rupture of an atherosclerotic plaque, it may also lead to vessel occlusion causing an ischemic stroke. The key player in hemostasis and thrombogenesis of arteries is the platelet. Therefore, antithrombotic treatment is the prime therapeutic option for prophylaxis and treatment of ischemic cerebrovascular disease.8) Alteration of blood flow volume and velocity is one of the most clinically important factors in thrombus formation. Arteries with stenosis show a markedly increased thrombogenic response. It is generally recognized that clinical atherosclerotic plaque or thrombus formation is frequently located near the site of stenosis or bifurcation of an artery. These findings suggest that introduction of a stenosis in these arteries enhances the interaction of platelets with the damaged arterial walls under abnormal fluid shear.11) If the mechanism of thrombosis is reduction of blood flow due to proximal stenosis of an artery, therapy for increasing blood velocity and volume such as angioplasty of the proximal stenosis can be a more important treatment. Posterior circulation infarcts account for approximately 25% of ischemic strokes.2) The most common mechanism is artery to artery embolism from vulnerable atheromatous plaques at the vertebral artery origin.1)
Free floating thrombi (FFT) has been reported as a rare cause of ischemic stroke. The natural history of FFT is not well known.3) Its etiology is considered multifactorial and it is usually associated with hypercoagulable disorders, such as hyperhomocystenemia, combined protein C and protein S deficiency, polycytemiavera, and systemic lupus erythematosus, in addition to its association with malignancy, trauma, and instrumentation.12) Transient hypercoagulable states and turbulent blood flow have been described as mechanistic etiologies. Igawa et al.11) reported that thrombus formation near the site of stenosis decreases with the decreasing degree of stenosis, whereas the percentage of thrombus formation in the distal region increases. Numerous mural platelet microthrombi are observed in the distal region of stenosed arteries. These findings suggest that introduction of a stenosis in these arteries enhances the interaction of platelets with the damaged arterial walls under abnormal fluid shear.11) High shear rates due to stenosis in arteries are a dominant cause of the thrombotic reaction.10)17) Treatment of FFT remains highly controversial. Medical treatments may lead to complete dissolution of the thrombus in most patients.15) The most popular medical management scheme was initial treatment with heparin, with conversion to warfarin anti-coagulation for several weeks to months. However, medical treatment (anticoagulant and thrombolysis) and endovascular treatment has been reported to increase the risk of distal embolism. Although medical treatments may lead particularly to complete dissolution of the thrombus in most patients, paradoxically there is a risk of systemic embolization by causing more rapid lysis of the pedicle compared with the thrombus itself. Surgical treatments (thrombectomy, thrombo-endarterectomy) carry a high risk of morbidity due to cerebral complication occurring in up to 29% of patients.3) Although both medical and surgical management have been successful, neither is clearly superior to the other. Various endovascular devices and treatment have recently been suggested. Several reports have been published on percutaneous embolectomy, which was performed using a protection device to reduce the risk of distal embolism.15) If the mechanism of thrombosis is reduction of blood flow due to proximal stenosis of an artery, therapy for increasing blood velocity and volume such as angioplasty of the proximal stenosis can be more effective than medical treatment.
We suggest that endovascular treatment, such as vertebral artery orifice stent and anti-platelet medication can be effective for FFT in an artery with proximal stenosis. However, we should consider that the patient with FFT usually requires appropriate anti-coagulation treatment instead of anti-platelet medication before endovascular or surgical treatment. We must strive to reduce the risk of distal migration of thrombus in treatment of FFT.
CONCLUSION
In patients with posterior circulation ischemic stroke who have VAH on initial contrast enhanced MRA, we can consider that VAH contributes to ischemic stroke. We describe an angiographic change of hypoplastic VA, including FFT with a vertebral artery origin stenosis after stent assisted angioplasty.
