Microsurgical Strategies Following Failed Endovascular Treatment with the Pipeline Embolization Device: Case of a Giant Posterior Cerebral Artery Aneurysm
Article information
Abstract
Treatment of giant posterior circulation aneurysms, via endovascular or microsurgical approaches, carries a high risk of morbidity and mortality. While flow-diverting stents (FDSs) represent a potent therapy for endovascular reconstruction of complex aneurysms, they are also associated with novel complications for which effective salvage techniques are lacking. We present a unique complication from failed treatment with a FDS. A 51 year-old male presented with increasing headaches secondary to a giant, fusiform aneurysm of the left posterior cerebral artery, which was largely thrombosed. Due to progressive enlargement of the aneurysm corresponding to worsening clinical symptoms, the lesion was treated with two Pipeline embolization devices (ev3, Plymouth, MN, United States). Three months after Pipeline embolization device treatment, complete posterior cerebral artery occlusion was observed at the origin of the proximal stent. Despite the lack of arterial inflow, the aneurysm dome continued to grow, resulting in obstructive hydrocephalus. Therefore microsurgical intervention was undertaken to trap and excise the aneurysm. The patient's postoperative course was complicated by multiple venous infarcts, ultimately resulting in death. Successful microsurgical obliteration of aneurysms previously treated with FDSs is extremely difficult. A combination of judicious preoperative planning and meticulous intraoperative surgical technique are requisite for effective management of these complicated cases.
INTRODUCTION
Giant intracranial aneurysms, defined as those greater than 25 mm in diameter, rank among the most complex neurosurgical pathologies to safely and effectively manage.6) Despite significant advances in microsurgical techniques and endovascular technology, successful obliteration of giant posterior circulation aneurysms remains very difficult and is associated with significant treatment-related morbidity and mortality.3)10) Flow-diverting stents (FDSs), such as the Pipeline embolization device (PED, ev3, Plymouth, MN, United States) and SILK stent (BALT Extrusion, Montmorency, France), have revolutionized the endovascular treatment of complex intracranial aneurysms.2) While these devices allow us to achieve new heights in endovascular aneurysm occlusion, they also present a new slate of complications for which the optimal management is not defined. The technical nuances of microsurgical intervention for aneurysms previously treated with a FDS have not previously been described. We present a case of giant posterior cerebral artery (PCA) aneurysm which underwent PED treatment but continued to enlarge, thereby necessitating microsurgical excision. We then discuss potential microsurgical salvage techniques for aneurysms which have failed endovascular cure with FDSs.
CASE REPORT
A 51 year-old male presented to an outside institution seven years prior with chronic headaches, which began following a motor vehicle accident. He had decreased visual acuity in the left eye from a previous gunshot wound to the face. The patient was diagnosed at initial presentation with a giant, fusiform left PCA aneurysm which was partially thrombosed. It was decided at the time of diagnosis that the risk of morbidity associated with either endovascular or microsurgical treatment of the aneurysm exceeded that of conservative management. Over time, the patient's headaches progressively worsened to an average visual analog scale (VAS) score of 7/10 despite maximal medical management, and the aneurysm gradually enlarged over serial angiographic imaging. The decision was made at this time to treat the lesion via an endovascular approach with the PED (Fig. 1). The aneurysm was successfully treated with two telescoping PEDs, 2.5×20 mm and 3.0×30 mm in size. The patient was discharged on standard oral dual antiplatelet therapy, clopidogrel 75 mg daily and aspirin 325 mg daily, without any change in neurological function. The severity of the patient's headache remained unchanged after PED treatment.
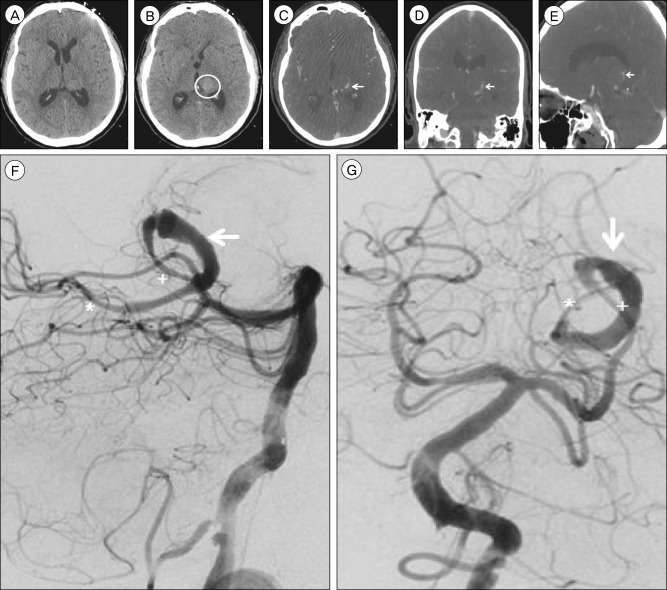
Non-contrast brain computed tomography (CT), axial view, (A) shows no evidence of obstructive hydrocephalus and (B) demonstrates a round, hyperdense structure (circle) in the region of the left thalamus compatible with the partially thrombosed aneurysm sac arising from the left posterior cerebral artery (PCA) previously diagnosed. The intra-aneurysmal thrombotic mass measures 21×21 mm, increased from 17×18 mm 10 weeks prior and from 14×14 mm eight months prior. Brain CT angiography (CTA), (C) axial, (D) coronal, and (E) sagittal views, demonstrates an irregular, fusiform dilatation of the left PCA P2 and P3 segments (arrow) with a largely thrombosed aneurysm sac arising medially. Cerebral angiography, lateral (F) and anteroposterior (G) views, demonstrates a giant, fusiform aneurysm of the left distal PCA P2 and P3 segments with a similar serpentine morphology (arrow) compared to prior angiography 10 months prior. The aneurysm measures approximately 40 mm in length and its proximal dilatation has increased in size to 4.5 mm from 3.7 mm on previous angiography. Arterial flow through the diseased segment of the parent artery is mildly delayed, and the majority of the aneurysm sac known to be thrombosed from non-invasive imaging (B-E). The diameter of the parent PCA vessel is 1.3 mm. The aneurysm was treated with two telescoping pipeline embolization devices, 2.5×20 mm and 3.0×35 mm in size, without complications. The proximal (+) and distal (*) ends of the dual PED construct are marked (F, G).
The patient presented three months post-PED with progressively worsening headaches to an average VAS score of 9/10, new gait ataxia, and new temporal hemianopsia of the right eye. While the stents remained patent at six weeks, by the follow-up angiography at three months, the diseased segment of the left PCA had progressed to complete occlusion at the origin of the proximal PED (Fig. 2). The patient's new temporal hemianopsia of the right eye was attributed to the left PCA occlusion. However, despite occlusion of the parent vessel, the aneurysm had continued to enlarge, resulting in local mass effect and cerebral edema as well as obstructive hydrocephalus. Therefore, the decision was made to surgically resect the aneurysm. The patient was taken to the operating room for an endoscopic third ventriculostomy, which was performed in a standard fashion through a right frontal burr hole without complications. An external ventricular drain was left in the frontal horn of the right lateral ventricle for subsequent cerebrospinal fluid drainage.
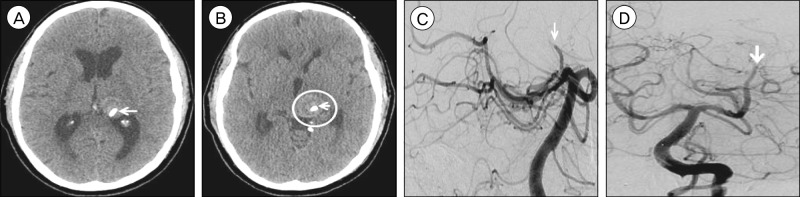
Non-contrast brain computed tomography (CT), axial view, performed six weeks after pipeline embolization device (PED) treatment demonstrates (A) interval development of obstructive hydrocephalus due to (B) enlargement of the hyperdense intra-aneurysmal thrombus (circle) to 24×24 mm from 21×21 mm previously, resulting in increased mass effect upon the posterior aspect of the third ventricle. The previously deployed PEDs (arrow) are visualized within the diseased parent artery (A, B). Cerebral angiography, lateral (C) and anteroposterior (D) views, performed three months after PED treatment demonstrates thrombosis of the left PCA at the P2 segment (arrow). The origin of the thrombosis corresponds to the origin of the proximal PED.
The patient was repositioned for aneurysm excision through an infratemporal, post-auricular, presigmoid skull base approach. Electrophysiological monitoring, including motor and somatosensory evoked potentials (motor evoked potential (MEP) and somatosensory evoked potential (SSEP), respectively), was utilized. A standard temporal craniotomy and partial mastoidectomy were performed. During the craniotomy, the sigmoid sinus was breached, requiring hemostatic control with gelfoam tamponade. After dural opening, 50 mL of cerebrospinal fluid was drained from the external ventricular drain to facilitate brain relaxation. After opening the arachnoid of the ambient cistern, the PCA and superior cerebellar artery were identified along with the fundus of the aneurysm dome posteriorly.
Due to the high degree of thrombosis, the aneurysm was firm and immobile. Therefore the aneurysm dome was opened so that the intrasaccular thrombus could be debulked. Decompression of the fundus facilitated dissection of the aneurysm from the surrounding cortex and brainstem. The proximally placed PED was identified in the lumen of the diseased PCA without evidence of endothelialization and was removed cautiously. After PED extraction from the inflow segment of the parent artery, there was brisk bleeding from the proximal PCA which controlled by clipping the P2 segment. After further debulking of the thrombus, the distal PED placed in the aneurysm outflow was identified and a clip was placed on the outflow portion of the PCA, effectively trapping the aneurysm. The entirety of the aneurysm was then excised from the PCA (Fig. 3). During the final dissection, the right-sided arm and leg SSEPs were lost without change in MEPs. At the end of surgery, there was no recovery of SSEPs in the right arm or leg and MEPs remained stable.
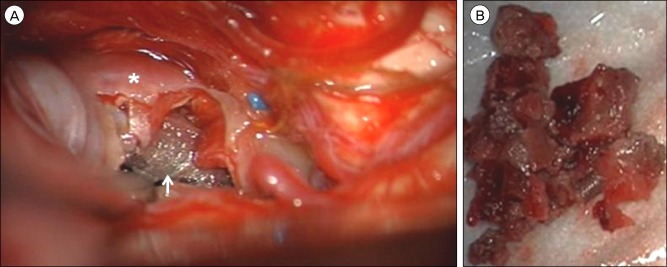
Following an infratemporal, post-auricular, presigmoid skull base approach to the middle fossa, the (A) aneurysm dome (asterisk) was visualized superior to the tentorial incisura through a subtemporal corridor. After the aneurysm dome was excised, the pipeline embolization device (arrow) was identified within the lumen of the diseased parent posterior cerebral artery. (B) Gross examination of the debulked thrombus removed from the aneurysm sac.
The patient's postoperative course was complicated by an extradural hematoma requiring surgical evacuation and thrombosis of the left transverse and sigmoid sinuses, resulting in multiple large territory venous infarcts requiring decompressive craniectomy. The extent of the postoperative infarcts was neurologically devastating, and the patient expired two weeks following the initial surgical intervention.
DISCUSSION
PCA aneurysms are rare, tend to affect a relatively younger patient population, and are more likely to be non-saccular than those located at more common sites.7) Based on large, prospective natural history studies of unruptured intracranial aneurysms, giant posterior circulation aneurysms are the subgroup associated with the highest rupture risk.1)8) Combined with the consideration that any treatment of giant posterior circulation aneurysms is associated with significant morbidity and mortality risks, the initial decision was made to manage the lesion conservatively, especially for a patient who only had headaches.3)10) The decision to intervene was made only when the patient's increasing headaches became refractory to medical management and associated with aneurysm enlargement, raising concern for impending rupture.
FDSs are a potent therapeutic option for complex aneurysms, but their applicability to aneurysms located distal to the supraclinoid internal carotid artery and those located in the posterior circulation remains controversial. Pistocchi et al. reported FDS treatment of 30 distally located aneurysms at or beyond the circle of Willis.11) The proportion of fusiform aneurysms was 23%, and the obliteration rate was 79% for lesions treated with FDS alone. The rate of permanent morbidity was 4% and there were no deaths, although no giant aneurysms were treated in the series. While the stents in our case were patent at six-week follow-up, the parent vessel was completely occluded three months after endovascular intervention. The patient did not suffer a large territory infarct, likely due to reconstitution of the distal PCA vascular supply via collateralization of pial and leptomeningeal vessels. Wajnberg et al. reported a similar case in a patient with a giant middle cerebral artery aneurysm.12) Despite the lack of arterial inflow, the aneurysm continued to enlarge. Even completely thrombosed aneurysms may continue to grow by parasitizing blood supply to the vasa vasorum of the aneurysm wall.4) A continuous cycle of mural hemorrhage and thrombosis by the vasa vasorum may result in progressive enlargement of occluded giant aneurysms. At this stage, these aneurysms begin to resemble neoplastic rather than vascular lesions. In our case, we did not visualize recruitment of the vasa vasorum in surgery. Additionally, we did not perform enhanced magnetic resonance imaging, which may have shown neuroimaging evidence of this phenomenon. Since cessation of arterial inflow was observed on post-PED angiography, we do not believe there was any influence of the PED on persistent aneurysm growth after occlusion of the parent artery.
Furthermore, there were no endovascular options at this point since occlusion of the parent artery rendered the aneurysm inaccessible from a transarterial route. Therefore, the only feasible strategy was microsurgical excavation of the aneurysm dome, given the patient's deteriorating neurological function and the obstructive hydrocephalus caused by the expanding aneurysm dome. Since there was no distal PCA flow on preoperative angiography, surgical reconstruction of the diseased parent vessel or distal bypass were not considered in our case. Unfortunately, due to poor collateral venous drainage, injury to the sigmoid sinus during the approach ultimately proved to be lethal. The patient's preoperative angiography showed limited collateral venous drainage. Therefore, we believe that sigmoid sinus occlusion by gelfoam tamponade was the principal cause of the venous infarctions. Another potential, although less likely, cause was prolonged temporal lobe retraction during surgery, which may have resulted in impaired cortical venous return or venous occlusion.
For giant and fusiform aneurysms such as the one we presented, traditional endovascular coiling treatments are ineffective.10) Coil embolization alone results in high recurrence rates for giant aneurysms and is not feasible for fusiform lesions. Parent artery occlusion, traditionally an effective endovascular technique for giant and fusiform aneurysms, cannot always be performed safely. Specifically, an aneurysm's parent artery cannot be sacrificed if the collateral vascular supply is inadequate to prevent an ischemic infarct. The FDS has changed the landscape of endovascular neurosurgery. Complex aneurysms previously impossible to safely occlude from an endovascular approach can now be successfully obliterated with FDSs.9) However, novel devices are accompanied by novel complications. We reported the microsurgical extraction of a malfunctioned PED which could not be retrieved by an endovascular approach following complete deployment.5) For an aneurysm which has failed FDS treatment, the endovascular options are relatively limited but include (1) placement of additional FDSs, (2) parent vessel occlusion with coils or a balloon without or (3) with a Wada test during balloon test occlusion, or (4) flow reversal in select cases. Microsurgical options are technically challenging and include (1) aneurysm clipping and parent vessel reconstruction with or without aneurysmorrhaphy, (2) wrapping of the aneurysm, (3) parent vessel occlusion with distal extracranial-intracranial bypass, and (4) aneurysm trapping and excision with distal bypass or in situ parent vessel reconstruction which would necessitate extraction of the intraluminal FDS. Due to the paucity of current literature describing these strategies, it is clear that future studies are needed to better define the most effective management strategies for complex aneurysms which have failed endovascular treatment with a FDS.
Based on our experience with the PED and the treatment of giant aneurysms, we believe that the use of FDSs should be very limited in perforator-rich arteries, such as the arteries comprising the circle of Willis, cerebral arteries distal to the circle of Willis, and arteries of the posterior circulation. In agreement with the literature, we found that the use of FDSs in perforator-rich arteries is associated with a significantly increased risk of thromboembolic complications. Therefore, alternative endovascular or surgical approaches should be considered for aneurysms arising from these locations before resorting to the use of FDSs. If surgery is undertaken, important countermeasures for undesirable intraoperative situations, such as major arterial or venous injury, include arterial re-anastomosis or bypass and repair or reconstruction of major venous sinuses.
CONCLUSIONS
This is the first reported case of PED extraction from a posterior circulation aneurysm and of microsurgical intervention following failed endovascular occlusion with a FDS. These cases are exceedingly difficult to manage successfully. Further experience and reports are necessary to define the optimal management of these patients.
