 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 25(3); 2023 > Article |
|
Abstract
Precise evaluation of the feeders, fistulous points, and draining veins plays a key role for successful embolization of intracranial dural arteriovenous fistulas (DAVF). Digital subtraction angiography (DSA) is a gold standard diagnostic tool to assess the exact angioarchitecture of DAVFs. With the advent of new image postprocessing techniques, we lately have been able to apply image fusion techniques with two different image sets obtained with flat panel detector rotational angiography. This new technique can provide additional and better pretherapeutic information of DAVFs over the conventional 2D and 3D angiographies. In addition, it can be used during the endovascular treatment to help the accurate and precise navigation of the microcatheter and microguidwire inside the vessels and identify the proper location of microcatheter in the targeted shunting pouch. In this study, we briefly review the process of an image fusion technique and introduce our clinical application for treating DAVFs, especially focused on the transvenous embolization.
The definition of intracranial dural arteriovenous fistulas (DAVF) is abnormal connections between the intracranial dural arteries and the venous system. The presence of cortical venous reflux (CVR) is the pivotal factor of current classification system because this is highly related with intracranial hemorrhage or other neurological deficits caused by venous congestion or infarction [4,10]. The DAVFs with these aggressive features should be aggressively treated [8,16,19]. For complete obliteration of DAVF without complications, one of the most important things is precise evaluation of multiple feeding arteries, the exact fistulous points, and the number and location of draining veins. In addition, we should thoroughly assess whether the draining venous sinus is used not only for shunt flow but also for normal venous circulation. Dangerous anastomoses between the internal and external carotid arteries should be also fully evaluated [13]. Digital subtraction angiography (DSA) is still gold standard diagnostic tool for identifying the exact angioarchitectures of DAVF before treatment. However, it is sometimes hard to find out the detailed angioarchitectures of DAVFs with conventional 2D or 3D angiography because of the overlapping vessels among the numerous feeders, multiple shunting points and draining veins [14,18]. With the development of angiography machines and image postprocessing workstations, we can make excellent fusion images using two different images sets which are reconstructed from 3D rotational angiography [7]. Previous investigators have reported the usefulness of an image fusion technique, which can provide various additional and better pretherapeutic information of intracranial vascular malformations for treatment planning [11,14,18]. In this study, we briefly review the process of an image fusion technique and introduce our clinical application for treating DAVFs, especially focused on the transvenous embolization.
Artis Zee (from May 2009 to February 2022) and Artis Icono (from March 2022 to present) biplane angiography machines (Siemens Healthcare, Germany) have been used to evaluate and treat DAVFs. We performed diagnostic six-vessel angiography under the local anesthesia in all cases with DAVFs before treatment and decided the treatment strategy based on the angiographic findings after the careful discussion between a neurovascular team composed of expert neurovascular neurosurgeons and an interventional neuroradiologist. Routine 2D angiographies of all six vessels including both internal carotid artery (ICA), both external carotid artery (ECA), and both vertebral artery (VA) were assessed in all cases. Dual rotational 3D angiographies were additionally conducted when feeders were arising from the vessel. By the postprocessing of dual rotational 3D angiography, we could obtain 3 different image sets named mask (bone only) in the first rotation, native fill (bone with vessel) in the second rotation, and subtraction (vessel only). Subtraction image was automatically reconstructed and formed by subtracting mask image out of native fill image. This series was named as DSA24, which emitted radiation dose of 0.24 ┬ĄGy/frame. It took 30 frame/s for 5 seconds in Artis Zee and 68 frame/s for 4 seconds in Artis Icono, leading to 133 projections in both machines. For contrast media, iodixanol (Visipaque 270, GE HealthCare, USA) was infused into the internal or external carotid artery at a rate of 2-4 mL/s for 5 seconds by injector with acquisition interval of 1-2 seconds based on the vessel diameter and shunt flow. Acquired images were transferred to workstation (syngo 3D workplace, Siemens, Germany) and the image postprocessing including image fusion technique were performed. After we registered two different mask images as a reference, any kind of two different image sets could be fused with each other in perfect alignment. We could obtain a total of six image sets from the two separated dual volume 3D rotational angiographies (Fig. 1) and make four different fusion images including (1) single vessel volume rendering (VR) fusion (mask in volume 1 with subtraction in volume 1), (2) single vessel multiplanar reconstruction (MPR) fusion, (3) dual vessel VR fusion (sub in volume 1 with sub in volume 2), and (4) dual vessel MPR fusion (native fill in volume 1 with native fill in volume 2) (Fig. 2).
Previously, we summarized our 10-year experiences of treating DAVFs. We have concluded that transvenous approach still have an important role even in transarterial Onyx era because transvenous embolization (TVE) would be more definitive modality for achieving complete obliteration [15]. TVE could be simpler and more straightforward if all cerebral venous system is fully connected with each other. However, we could encounter some cases with occluded accessing routes such as isolated sinus and inferior petrosal sinus (IPS) occlusion in DAVFs involving cavernous sinus DAVF (CSDAVF). We can try to breach the occluded routes by blinded navigation with microguidewire and microcatheter. This method is usually useful and effective [2,6]. But it is not always feasible and sometimes results in serious complications including vessel perforation [6]. Moreover, blinded navigation can make the microcatheter headed for the wrong direction during the procedure. Head and neck veins have complex anatomy. There are numerous tributaries and confluences that can hinder smooth microcatheter advancement to the target shunt pouch [1,3,9]. Some specific locations such as anterior condylar confluence (ACC) DAVF is challenging for transvenous approach because of the complex anatomy around the hypoglossal canal [5,17]. Additionally, roadmap images during the venous approach are not sometimes handy because of its low quality caused by an overlap of numerous feeding arteries, shunting pouch, and draining veins [7]. The one of the most important factors for successful TVE of DAVFs is the safe and accurate placement of microcatheter for coil delivery to the shunt pouch over these obstacles. To overcome all the hurdles during TVE, we can use intraprocedural image techniques to make the blind navigation of the microcatheter inside the complex head and neck veins easier and to verify the exact placement of the microcatheter to the target pouch.
An image fusion technique can be used not only for pretherapeutic precise evaluation but also for the intraprocedural application of delivering the microcatheter appropriately into the targeted shunt pouch. After the placement of 5 Fr angio-catheter in the arterial side and 7 Fr guiding catheter in the venous side under the general anesthesia, we initially performed DSA24 series with contrast injection into the artery and reconstructed image sets. During the navigation of microcatheter with microguidewire, additional low dose, single-rotational 3D angiographies named as DRcare (0.10 ┬ĄGy/frame for 4-5 seconds) with reduced field of view (FOV) without contrast injection were conducted whenever it was needed. These images were obtained with keeping the microcatheter and microguidewire inside the vessels and fused with initial DSA24 to confirm the direction of the microcatheter during the navigation and the proper location of the microcatheter in the target before starting endovascular embolization. Thereafter, detachable and/ or pushable coils were inserted until the fistulous points obliterated.
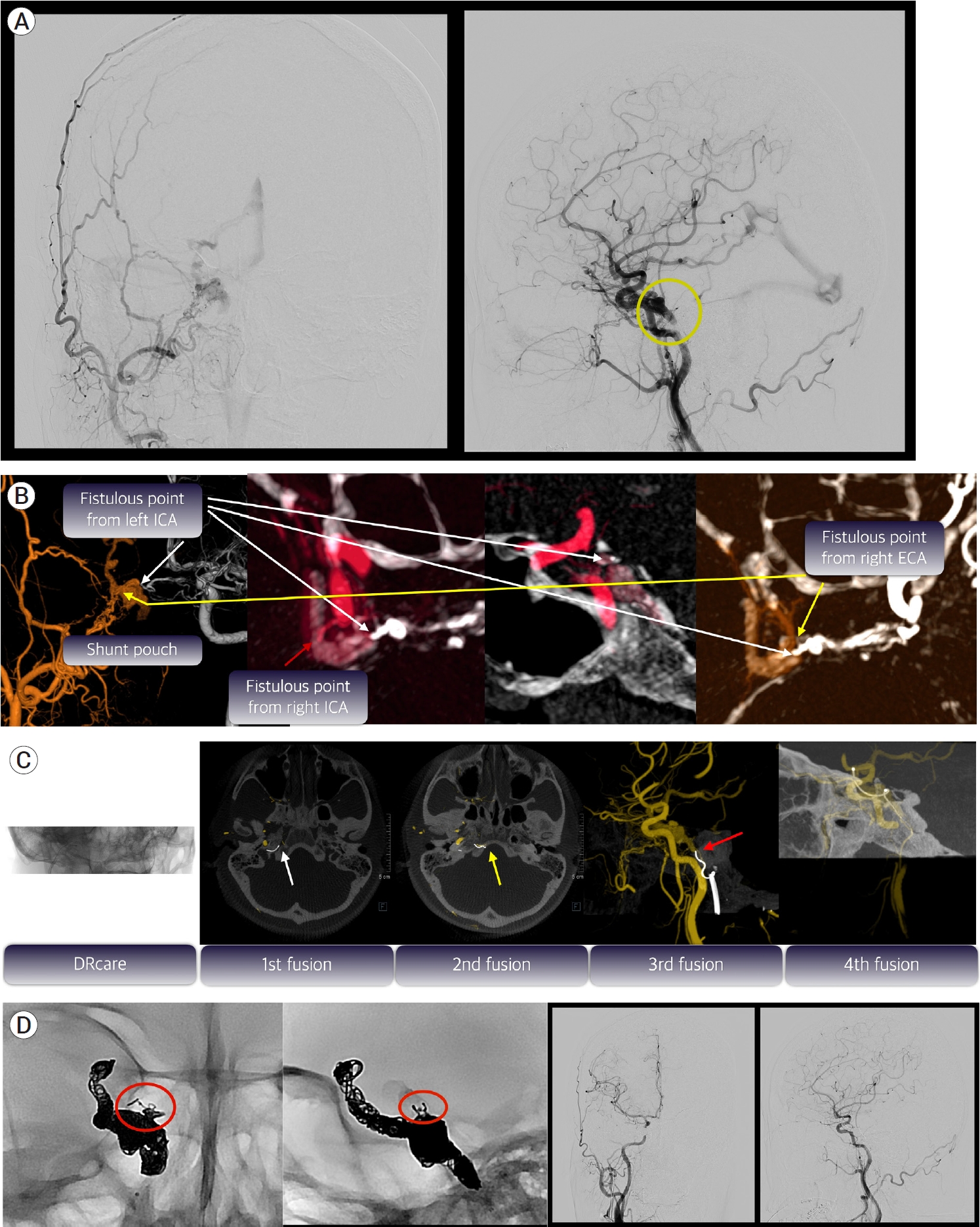
A 44-year-old female presented with conjunctival injection on her right eye. Diagnostic 6 vessel angiography showed DAVF involving right CS fed by both ICA and right ECA and drained only to straight sinus via basal vein of Rosenthal (Fig. 3A). The exact fistulous point located in the superomedial portion of CS was identified by use of various fusion images (Fig. 3B). Before starting the microcatheter navigation through the vein, we obtained DSA24 series as a reference. During the blinded navigation for breaching the occluded inferior petrosal sinus (IPS), we checked additional DRcare series and fused with initial DSA24 to identify whether the microcatheter headed for the right direction. After conducting DRcare 4 times, we eventually delivered the microcatheter into the target reflux vein through the CS (Fig. 3C). Thereafter, we inserted coils from the cortical vein to CS in order and achieved complete obliteration (Fig. 3D).
Even in use of image fusion technique, there are still several difficulties of transvenous approach for DAVF. The specific anatomical features of head and neck vein including abrupt angulation and tortuous course attribute some troubles for microcatheter navigation. In this situation, we could effectively navigate inside the veins using microguidewire looping technique as we previously reported [9]. Another drawback of transfemoral transvenous approach is the instability of the proximal guiding catheter. The guiding catheter is usually weakly located in the vein because of the larger diameter and weaker wall tension compared to the arteries [12]. Because strong proximal support is mandatory for successful TVE, we placed large diameter guiding catheter in the venous system as distally as possible or used coaxial system using 5 Fr angiocatheter or intermediate catheter if needed.
Intraprocedural image fusion technique using flat panel detector rotation angiography is very useful 1) to identify the microcatheter tip location with bony landmark during the blinded navigation, 2) to aid an appropriate navigation of microcatheter inside the complex venous structures, and 3) to confirm the proper placement of microcatheter in the targeted shunt pouch. Therefore, our technique would be more effective for transvenous embolization of DAVFs with occluded accessing route or involving skull base around hypoglossal canal.
Fig.┬Ā1.
Algorithm to make various fusion images. VR, volume rendering; MPR, multiplanar reconstruction
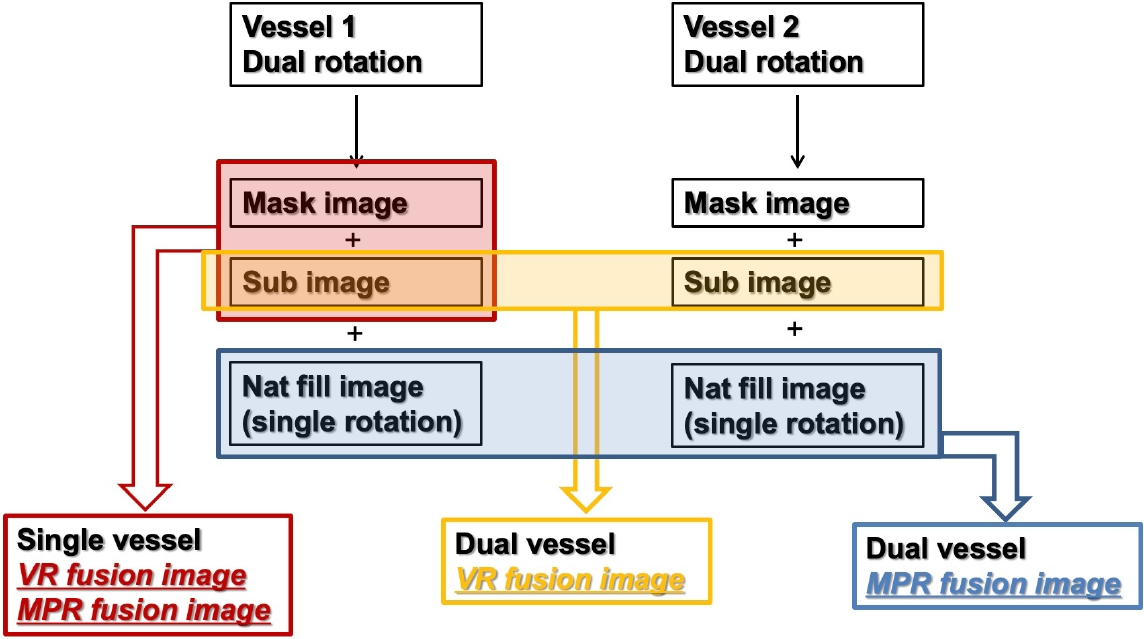
Fig.┬Ā2.
Example of various fusion images. (A) Single vessel volume rendering (VR) fusion image with mask of vessel 1 and sub of vessel 1. (B) Single vessel multiplanar reconstruction (MPR) axial fusion image with mask of vessel 1 and sub of vessel 1. (C) Dual vessel VR fusion image with sub of vessel 1 and sub of vessel 2. (D) Dual vessel MPR fusion image with nat fill of vessel 1 and nat fill of vessel 2.
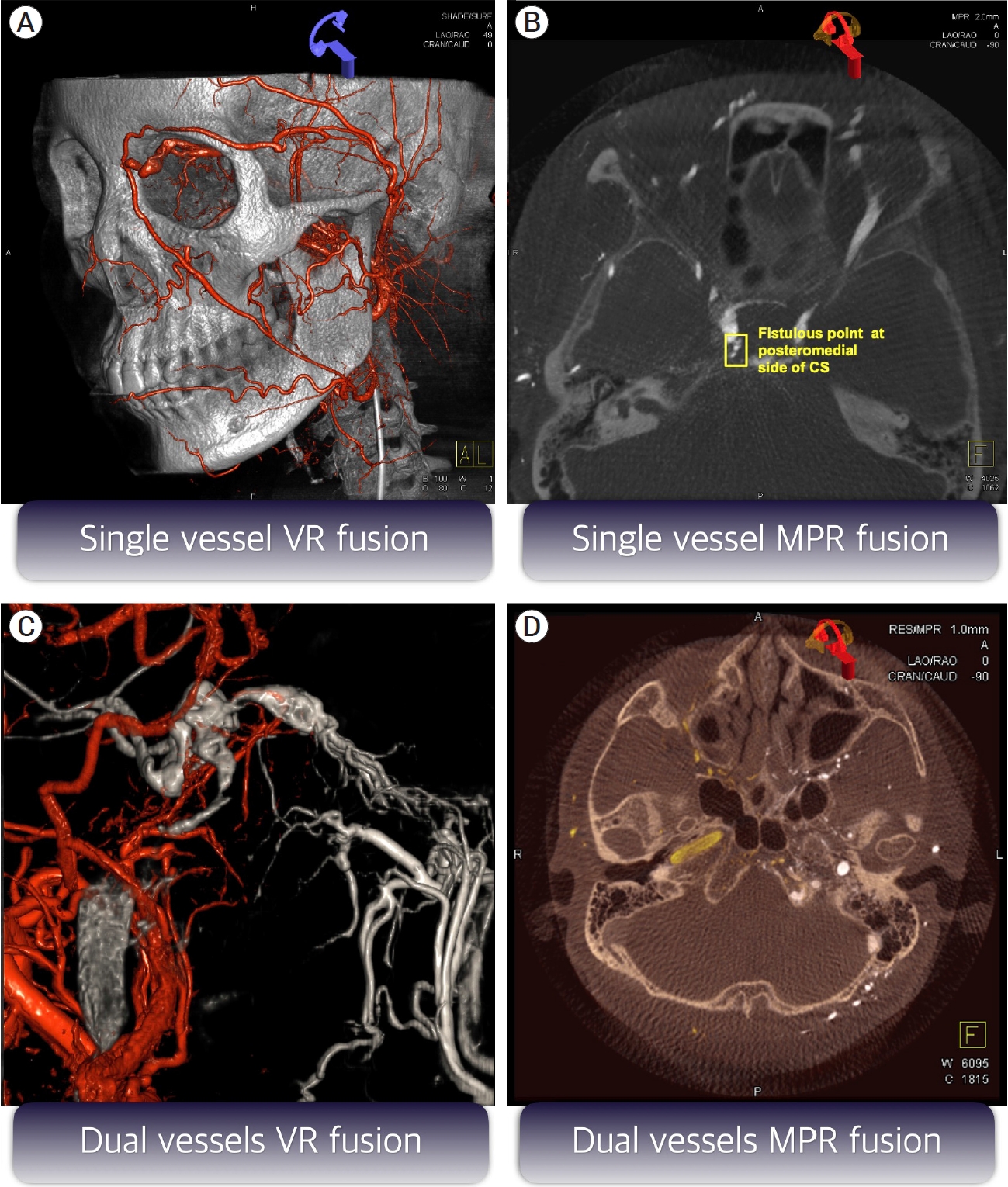
Fig.┬Ā3.
Illustrative case of 44-year-old woman with conjunctival injection and periorbital swelling of her right eye. (A) Initial DSA showed dural arteriovenous fistula (DAVF) involving right cavernous sinus (CS) with the cortical venous reflux (CVR) and the occluded inferior petrosal sinus (IPS, yellow circle). (B) By use of pretherapeutic various fusion images, the fistulous point was exactly identified. Even though there were numerous feeders arising from right external carotid artery (ECA, yellow arrow), right internal carotid artery (ICA, red arrow), and left ICA (white arrow), the almost all feeders converged on the superomedial portion of the CS. (C) First intraprocedural fusion image with DRcare and initial DSA24 showed that the microcatheter tip entered the groove for IPS (white arrow). Second fusion image obtained after more advancing the microcatheter demonstrated the wrong direction of the microcatheter tip (yellow arrow). Third fusion image showed that the microguidwire was placed just below the entry point of the occluded CS (red arrow). The microcatheter was finally delivered into the target reflux vein through the CS and this was verified by 4th fusion image. (D) We put the coils from cortical vein to the target fistulous point (red circle) located in superomedial portion of the CS in order and could eventually achieve complete obliteration. DSA, Digital subtraction angiography
REFERENCES
1. Alexandre AM, Visconti E, Lozupone E, DŌĆÖArgento F, Pedicelli A. Embolization of dural arteriovenous fistula of the cavernous sinus through percutaneous ultrasound-guided puncture of the facial vein. World Neurosurg. 2017 Mar;99:812.

2. Benndorf G, Bender A, Lehmann R, Lanksch W. Transvenous occlusion of dural cavernous sinus fistulas through the thrombosed inferior petrosal sinus: Report of four cases and review of the literature. Surg Neurol. 2000 Jul;54(1):42-54.


3. Biondi A, Milea D, Cognard C, Ricciardi GK, Bonneville F, van Effenterre R. Cavernous sinus dural fistulae treated by transvenous approach through the facial vein: Report of seven cases and review of the literature. AJNR Am J Neuroradiol. 2003 Jun-Jul;24(6):1240-6.


4. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995 Feb;82(2):166-79.


5. Caton MT, Narsinh KH, Baker A, Dowd CF, Higashida RT, Cooke DL, et al. Dural arteriovenous fistulas of the foramen magnum region: Clinical features and angioarchitectural phenotypes. AJNR Am J Neuroradiol. 2021 Aug;42(8):1486-91.



6. Cho YD, Rhim JK, Yoo DH, Kang HS, Kim JE, Cho WS, et al. Transvenous microguidewire looping technique for breach of ipsilateral inferior petrosal sinus occlusions en route to cavernous sinus dural arteriovenous fistulas. Interv Neuroradiol. 2016 Oct;22(5):590-5.




7. Choi JH, Cho DY, Shin YS, Kim BS. Intraprocedural flat panel detector rotational angiography and an image fusion technique for delivery of a microcatheter into the targeted shunt pouch of a dural arteriovenous fistula. AJNR Am J Neuroradiol. 2020 Oct;41(10):1876-8.



8. Choi JH, Jo KI, Kim KH, Jeon P, Yeon JY, Kim JS, et al. Early rebleeding of intracranial dural arteriovenous fistulas after an intracranial hemorrhage. Acta Neurochir (Wien). 2017 Aug;159(8):1479-87.



9. Choi JH, Shin YS, Kim BS. Making microguidewire loop facilitates navigation through tortuous or abruptly angulated head and neck veins to access cavernous sinus dural arteriovenous fistulas. World Neurosurg. 2019 Sep;129:e561-5.


10. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995 Mar;194(3):671-80.


11. Fukuda K, Higashi T, Okawa M, Iwaasa M, Abe H, Inoue T. Fusion technique using three-dimensional digital subtraction angiography in the evaluation of complex cerebral and spinal vascular malformations. World Neurosurg. 2016 Jan;85:353-8.


12. Karygiannis MN, Szatmary Z, Claudino PA, Houdart E. Facial vein catheterization for transvenous embolization of the cavernous sinus. Technique and advantages of the direct jugular vein approach - Report of three cases. Interv Neuroradiol. 2006 Mar;12(1):25-30.




13. Kuwayama N, Akioka N. Complications of endovascular treatment of intracranial dural arteriovenous fistulas. Acta Neurochir Suppl. 2021 132:123-7.


14. Li H, Wan F, Li J, Sheng L, Li G, Chen G, et al. Flat detector computed tomography-based ŌĆ£dual vessel fusionŌĆØ technique for diagnosis and surgical planning in the management of dural arteriovenous fistula. World Neurosurg. 2015 Aug;84(2):520-7.


15. Oh SH, Choi JH, Kim BS, Lee KS, Shin YS. Treatment outcomes according to various treatment modalities for intracranial dural arteriovenous fistulas in the Onyx era: A 10- year single-center experience. World Neurosurg. 2019 Jun;126:e825-34.


16. S├Čderman M, Pavic L, Edner G, Holmin S, Andersson T. Natural history of dural arteriovenous shunts. Stroke. 2008 Jun;39(6):1735-9.


17. Spittau B, Mill├Īn DS, El-Sherifi S, Hader C, Singh TP, Motschall E, et al. Dural arteriovenous fistulas of the hypoglossal canal: Systematic review on imaging anatomy, clinical findings, and endovascular management. J Neurosurg. 2015 Apr;122(4):883-903.


- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,128 View
- 38 Download
- ORCID iDs
-
Jai Ho Choi

https://orcid.org/0000-0001-6838-0343 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



