 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 18(3); 2016 > Article |
|
Abstract
Intracranial pial arteriovenous fistulas (AVFs) are rare cerebrovascular lesions consisting of one or more arterial connections to a single venous channel without an intervening nidus. Because of the location and high flow dynamics of these lesions, neurosurgeons may have a difficulty deciding between endovascular treatment and open surgical treatment. We report on a patient who underwent endovascular treatment with liquid embolic agent. A 50-year-old man with a decreased mental state and a tonic seizure event was brought to our hospital. Computed tomography (CT) of the brain showed a subcortical hematoma in the right temporoparietal lobe. On three-dimensional cerebral artery CT, there was no evidence of definite cerebrovascular abnormality. Cerebral angiography showed a pial AVF supplied by the right middle cerebral artery with early drainage into the right superior cerebral vein. The patient was treated with Onyx embolization for definitive closure of the fistula. The patient was transferred to the department of rehabilitation medicine two weeks later with grade 4 left hemiparesis. The application of advanced equipment, such as the latest angiography and endovascular tools, will facilitate the correct diagnosis and delicate treatment of pial AVF.
Intracranial pial arteriovenous fistula (AVF) is a rare cerebrovascular malformation, accounting for only 1.6% of all vascular malformation of the brain.9)10) Pial AVF was once regarded as a type of arteriovenous malformation (AVM). However, current evidence suggests that the pathological characteristics and clinical presentations of pial AVF, as well as its therapeutic options, are different from those of AVM.23) Pial AVF has a single or multiple arterial connections to single venous channel without any intervening nidus. Pial AVF differs from dural AVF in that the arterial supply is derived from pial or cortical arterial vessel and the location is not within the dural leaflets.10) Because of high flow dynamics, pial AVF is often associated with a venous varix.16) The majority of pial AVF is considered congenital, and it is well known to be associated with congenital hereditary vasculopathies.7)
Disconnection of the arteriovenous shunt is sufficient to obliterate the lesion, so lesion resection is unnecessary. We describe a case of pial AVF with intracranial hematoma treated with Onyx embolization.
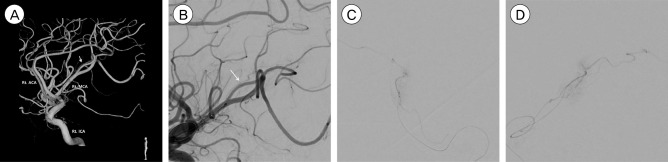
A 50-year-old man with a decreased mental state and a tonic seizure event was brought to our hospital. He had no past medical history and no history of head trauma. On initial neurological examination, he was in a drowsy state (Glasgow Coma Scale score of E3 V3 M6) with grade 2 left hemiparesis. Computed tomography (CT) of the brain showed a subcortical hematoma measuring approximately 7 mL in the right temporoparietal lobe (Fig. 1). On three-dimensional cerebral artery CT performed to evaluate the cause of the hemorrhage, there was no evidence of a definite vascular abnormality. Cerebral angiography (Philips Allura Clarity FD 20/20, Best, Netherlands) was performed for further evaluation of vascular malformation and other causes of the hemorrhage. Cerebral angiography showed a suspicious AVF. We performed super-selective angiography via utilization of a micro-catheter, and identified a pial AVF supplied by the distal branch of the inferior division of the right middle cerebral artery, with early drainage into the right superior cerebral vein (Fig. 2).
Under general anesthesia, the embolization procedure was performed using MarathonTM micro-catheter (Covidien, Irvine, CA, USA). The working micro-catheter was primed with normal saline and dimethyl sulfoxide (DMSO) in the standard fashion. A total of approximately 0.1cc of Onyx 34 (8% ethylene vinyl alcohol copolymer, Covidien, Irvine, CA, USA) was infused slowly under continuous fluoroscopic visualization. Angiography obtained through a guide-catheter injection showed that the fistula had been obliterated and the Onyx cast was seen stable. Final angiography after micro-catheter was removed showed no arteriovenous shunting (Fig. 3).
The patient's mental state recovered to alertness, and his left-sided motor strength was improved. He was transferred to the department of rehabilitation medicine two weeks later for active rehabilitation.
Intracranial pial AVF is a rare cerebrovascular lesion that for decades was considered a variant of AVM. However, subsequent studies have revealed that pial AVF is a distinct entity from cerebral AVM, dural AVF, or other cerebral vascular lesions due to the different angiographic findings, clinical course, and therapeutic options for pial AVF.13) The abnormality from a pial AVF arises from its high-flow nature. Communication between an arterial feeder directly into a solitary draining vein without an intervening tangle of vessels creates conditions for rapid high flow. Associated venous varices are produced by the high and turbulent flow from arteriovenous shunting.1)3)4)5)8) Due to lack of intervening of vessels, there is higher pressure gradient, which can make such lesions more vulnerable to rupture, leading to a poor prognosis.2)
Pial AVF can result from trauma17) or may be congenital. These lesions are more common in children and are frequently associated with hereditary hemorrhagic telangiectasia.12)22) It is possible that a misstep in embryological development of the cerebrovasculature produces these lesions. Alternatively, abnormal angiogenesis and associated vascular growth factors and cytokines may play important roles.10)
Due to their rarity, little is known about the natural history of intracranial pial AVFs. However, it could be dangerous to leave the fistula untreated. One study reported an association between conservative management of pial AVF and high mortality: five (63%) of eight patients who received conservative management died due to acute or subsequent fatal bleeding. Therefore, due to its unfavorable natural course, pial AVF requires surgical or endovascular treatment.15)
Patients with pial AVF lesions can present with hemorrhage, seizure, headache, high-output cardiac failure in neonates and infants, macrocephaly, neurological deficits, intracranial bruit, and symptoms of increased intracranial pressure.6)14)18)19) The manifestations of pial AVF vary according to patient's age and the presence of a varix. In one report, younger patients (< 15 years old) were more likely to have a varix and symptoms related to shunting effect, while hemorrhage was the main presentation in older patients.23)
Treatment of pial AVF differs from that of AVM. Because there is no nidus, disconnection of the arteriovenous communication could eliminate the abnormality without the necessity of lesion resection. In AVM, resection of the entire lesion is necessary because of the multiplicity of the communicating shunts. Occlusion of only the feeding arteries of an AVM leaves behind the nidus, which can recruit new arterial feeders that are often even more difficult to access by transarterial embolization. In addition, in AVM, the draining veins must be obliterated because they may persist after resection and may recruit recommunication with collaterals. The pathological features of a congenital pial AVF arise principally from its high-flow nature. Treatment of pial AVF by simply cutting off the shunt connection through surgery or endovascular intervention is sufficient, with removal of the entire vascular malformation was unnecessary.10)24)25) Disconnection of the high-flow system leads to elimination of the abnormality and its accompanying elements, such as venous varices.
Disconnection of the arteriovenous shunting has traditionally been accomplished surgically by either clip application or cauterization of the vessel. These method have been has proven effective. However, some lesions may be in deep or surgically inaccessible locations and are thus associated with high surgical risk. Recent endovascular applications have been reported as successful means of disconnecting AVF with a variety of different agents such as balloons, coils, glue, silk sutures, or polyvinyl alcohol.19)20)21) Accessing the lesion via an endovascular route avoids the risks associated with craniotomy and surgical-dissection approach to the abnormality. However, endovascular attempts are not always successful or safe. High-flow lesions can make it difficult to deliver the desired embolic agent precisely to the fistulous point. Delivery of embolic material into the draining vein can compromise venous outflow of the fistula or cause pulmonary embolus. Restricted venous outflow in the setting of an incompletely obliterated fistula can have disastrous consequences.8)11)15)18)19) Newman et al.16) reported that balloons have been used successfully to modulate and arrest the flow of feeding artery. It is therefore important to choose the safer and more effective therapeutic method in a case-by-case manner.
As endovascular technologies continue to rapidly advance, we expect higher pial AVF detection rates, as well as higher success rates with endovascular flow disconnection of arteriovenous shunting.
Pial AVFs are rare intracranial vascular malformations, and due to their poor natural course and significant morbidity/mortality, prompt diagnosis followed by appropriate treatment is very important. With recent advancement in micro-catheters, embolic agents (such as onyx), and other modalities (such as recent interventional x-ray system), the endovascular approach can be a delicate and safe procedure for the treatment of pial AVF.
References
1. Almeida GM, Shibata MK. Hemispheric arteriovenous fistulae with giant venous dilation. Childs Nerv Syst. 1990 6;6(4):216-219;



2. Alurkar A, Karanam LS, Nayak S, Ghanta RK. Intracranial pial arteriovenous fistulae: diagnosis and treatment techniques in pediatric patients with review of literature. J Clin Imaging Sci. 2016 1;6:2



3. Aoki N, Sakai T, Oikawa A. Intracranial arteriovenous fistula manifesting as progressive neurological deterioration in an infant: case report. Neurosurgery. 1991 4;28(4):619-622; discussion 622-3.



4. Barnwell SL, Ciricillo SF, Halbach VV, Edwards MS, Cogen PH. Intracerebral arteriovenous fistulas associated with intraparenchymal varix in childhood: case reports. Neurosurgery. 1990 1;26(1):122-125;


5. Carrillo R, Carreira LM, Prada J, Rosas C, Egas G. Giant aneurysm arising from a single arteriovenous fistula in a child. Case report. J Neurosurg. 1984 5;60(5):1085-1088;


6. Garcia-Monaco R, De Victor D, Mann C, Hannedouche A, Terbrugge K, Lasjaunias P. Congestive cardiac manifestations from cerebrocranial arteriovenous shunts. Endovascular management in 30 children. Childs Nerv Syst. 1991 2;7(1):48-52;



7. García-Mónaco R, Taylor W, Rodesch G, Alvarez H, Burrows P, Coubes P, et al. Pial arteriovenous fistula in children as presenting manifestation of Rendu-Osler-Weber disease. Neuroradiology. 1995 1;37(1):60-64;



8. Giller CA, Batjer HH, Purdy P, Walker B, Mathews D. Interdisciplinary evaluation of cerebral hemodynamics in the treatment of arteriovenous fistulae associated with giant varices. Neurosurgery. 1994 10;35(4):778-782; discussion 782-4.



9. Halbach VV, Higashida RT, Hieshima GB, Hardin CW, Dowd CF, Barnwell SL. Transarterial occlusion of solitary intracerebral arteriovenous fistulas. AJNR Am J Neuroradiol. 1989 Jul-Aug;10(4):747-752;


10. Hoh BL, Putman CM, Budzik RF, Ogilvy CS. Surgical and endovascular flow disconnection of intracranial pial single-channel arteriovenous fistulae. Neurosurgery. 2001 12;49(6):1351-1363; discussion 1363-4.



11. Kikuchi K, Kowada M, Sasajima H. Vascular malformations of the brain in hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease). Surg Neurol. 1994 5;41(5):374-380;


12. Krings T, Ozanne A, Chng SM, Alvarez H, Rodesch G, Lasjaunias PL. Neurovascular phenotypes in hereditary haemorrhagic telangiectasia patients according to age. Review of 50 consecutive patients aged 1 day-60 years. Neuroradiology. 2005 10;47(10):711-720;



13. Lasjaunias P, Manelfe C, Chiu M. Angiographic architecture of intracranial vascular malformations and fistulas--pretherapeutic aspects. Neurosurg Rev. 1986 9(4):253-263;



14. Lv X, Li Y, Jiang C, Wu Z. Endovascular treatment of brain arteriovenous fistulas. AJNR Am J Neuroradiol. 2009 4;30(4):851-856;



15. Nelson PK, Niimi Y, Lasjaunias P, Berenstein A. Endovascular embolization of congenital intracranial pial arteriovenous fistulas. Neuroimaging Clin N Am. 1992 2(2):309-317.
16. Newman CB, Hu YC, McDougall CG, Albuquerque FC. Balloon-assisted Onyx embolization of cerebral single-channel pial arteriovenous fistulas. J Neurosurg Pediatr. 2011 6;7(6):637-642;


17. Nomura S, Ishikawa O, Tanaka K, Otani R, Miura K, Maeda K. Pial arteriovenous fistula caused by trauma: a case report. Neurol Med Chir (Tokyo). 2015 55(11):856-858;



18. Tomlinson FH, Rüfenacht DA, Sundt TM Jr, Nichols DA, Fode NC. Arteriovenous fistulas of the brain and the spinal cord. J Neurosurg. 1993 7;79(1):16-27;


19. Vinuela F, Drake CG, Fox AJ, Pelz DM. Giant intracranial varices secondary to high-flow arteriovenous fistulae. J Neurosurg. 1987 2;66(2):198-203;


20. Viñuela F, Fox AJ, Kan S, Drake CG. Balloon occlusion of a spontaneous fistula of the posterior inferior cerebellar artery. Case report. J Neurosurg. 1983 2;58(2):287-290;


21. Wang YC, Wong HF, Yeh YS. Intracranial pial arteriovenous fistulas with single-vein drainage. Report of three cases and review of the literature. J Neurosurg. 2004 2;100(2 Suppl Pediatrics):201-205;

22. Weon YC, Yoshida Y, Sachet M, Mahadevan J, Alvarez H, Rodesch G, et al. Supratentorial cerebral arteriovenous fistulas (AVFs) in children: review of 41 cases with 63 non choroidal single-hole AVFs. Acta Neurochir (Wien). 2005 1;147(1):17-31; discussion 31.



23. Yang WH, Lu MS, Cheng YK, Wang TC. Pial arteriovenous fistula: a review of literature. Br J Neurosurg. 2011 10;25(5):580-585;


Fig. 1
A CT scan on admission showing a subcortical ICH on right temporo-parietal lobe. CT = computed tomography; ICH = intracranial hematoma.
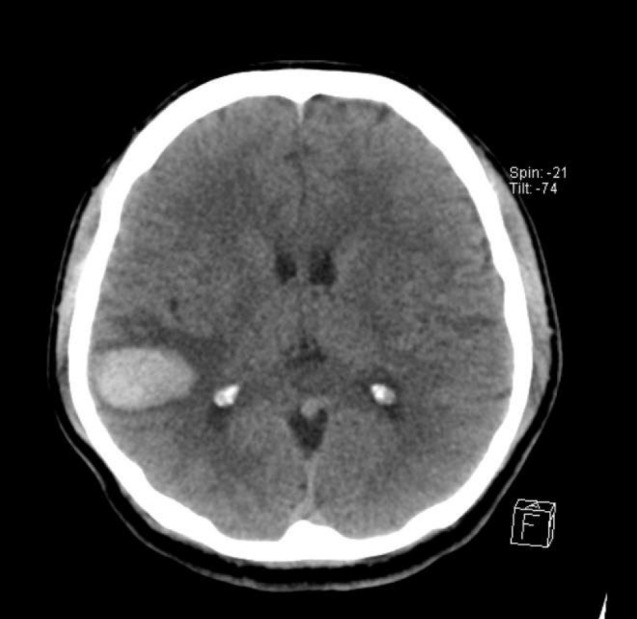
Fig. 2
(A) Three dimensional reconstruction of the right ICA angiography. A thin arteriovenous fistula is shown (white arrow). (B) A magnified angiography of right ICA, lateral view. (C, D) Super-selective micro-catheter angiography of right distal middle cerebral artery confirms a pial arteriovenous fistula supplied by the distal branch of inferior division of right distal MCA. ACA = anterior cerebral artery; MCA = middle cerebral artery; ICA = internal carotid artery.
- TOOLS
-
METRICS

-
- 6 Crossref
- 0 Scopus
- 2,308 View
- 24 Download
- Related articles
-
Role of surgery in management of intracranial dural arteriovenous fistulas2023 June;25(2)
Management of Intracranial Cavernous Malformations.1999 March;1(1)





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



