 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 21(2); 2019 > Article |
|
Abstract
Objective
Identifying collaterals from external carotid artery (ECA) is necessary before treatment of ophthalmic artery (OphA) aneurysm. We present a manual carotid compression test to verify collaterals in ophthalmic artery aneurysms, and evaluate its usefulness.
Materials and Methods
From March 2013 to December 2017, endovascular coiling was performed 19 consecutive patients with 20 OphA aneurysms. We performed manual carotid compression test for patients who had aneurysms incorporating entry of OphA. Clinical and angiographic outcomes were investigated.
Results
Of 13 cases underwent manual carotid compression test, 12 cases were confirmed collateral flow from ECA to OphA. During the coil embolization, we tried to maintain the original OphA flow even if it has a collateral anastomosis. Among them, OphA occlusion occurred in one patient during coiling. Recurrence of aneurysm was occurred in a ruptured case and additional embolization was required.
Visual complication is major concern when coiling ophthalmic artery (OphA) aneurysms. Fortunately, collateral flow through external carotid artery (ECA) is often well developed in OphA.11)15)17)18) If ECA collaterals are sufficient, visual complication would not occur even if OphA is occluded.1)7) Therefore, verifying ECA collaterals of OphA is crucial before the treatment in case of an aneurysm incorporating an OphA origin.
Balloon test occlusion (BTO) is usually used to identify collateral flow of OphA. However, there are some reports of severe complication related with BTO, such as dissection, aneurysm rupture and thromboembolism.2)14) In addition, it is difficult to perform in settings of routine cerebral angiography, because BTO needs to more steps to perform, including installing guiding catheter and intracranial procedure.
Thus, we present a simple manual carotid compression test which can simultaneously perform during cerebral angiography, as alternative to BTO, in OphA aneurysms and investigate its usefulness.
From March 2013 to December 2017, 1206 patients were treated with intracranial aneurysms at our institution. Among them, we identified 20 OphA aneurysms in 19 patients from the retrospectively recorded data base. The OphA was classified by the degree to which the OphA was incorporated by the aneurysm and the contiguity between the OphA entry and parent ICA. Aneurysms incorporating an OphA origin were defined as OphA type, and those adjacent to the OphA but originating from the ICA were termed ICA type.1) We performed the manual carotid compression test in case of only OphA type.
We performed digital subtraction angiography (DSA) on a biplane angiographic system (Allura, Philips Healthcare, The Netherlands). Basically, we acquired anteroposterior and lateral images of two-dimensional DSA from internal carotid artery (ICA). After that we performed rotational angiography at affected side of ICA. We added two-dimensional DSA of profile angle to identify the relationship between the aneurysm and the OphA. When an OphA aneurysm was confirmed the OphA type aneurysms, we added ECA angiography and compression test to evaluate collateral flow.
Before the manual carotid compression test, firstly we confirmed presence of atheroma near the carotid bulb through common carotid artery (CCA) angiography and if a patient had it, we did not perform manual compression. Small-sized aneurysms not planned for treatment were also not performed the manual compression test.
We identified a location of proximal ICA through the CCA angiography, by comparing with anatomical landmark of neck, such as thyroid cartilage and hyoid bone. After performing routine ECA angiography, kept 5-French angiographic catheter in the distal ECA as far away as possible beyond the origin of facial artery. And then ipsilateral submandibular area was palpated to find ICA. When the ICA location was confirmed, ECA angiography was performed immediately after starting manual compression of ipsilateral ICA. We observed both anteroposterior and lateral DSA images to verify collateral flow from ECA to OphA. If choroidal blush or retrograde blood flow of OphA was seen, we considered it as positive collaterals (Fig. 1). If the collateral flow did not appear at first attempt, we repeated the test up to two more times. If the choroidal blush or retrograde flow of OphA is not visible through three tests, we considered it as negative.
After the cerebral angiography and the manual carotid compression test, we performed neurological examination to confirm the test-related complication.
All patients were treated by coil embolization under the general anesthesia. In patients with an unruptured aneurysm, dual antiplatelet agents (100mg aspirin and 75mg clopidogrel) were administered daily for 1 week before coiling. During procedure, loading dose of 3000IU heparin was given intravenously immediately after the femoral sheath was placed and added 1000IU every hour. In patients with a ruptured aneurysm, procedures were performed by emergency on the day of admission. The antiplatelet agent was not administered before coiling and intravenous heparin of 2000IU was given after near-complete obliteration of the ruptured aneurysm. If the aneurysm had an unfavorable morphology for simple coiling, we used double catheter technique or stent-assisted technique. After the procedure, if the patient who treated by stent assisted coil embolization, dual antiplatelet agents were prescribed at least 6 months. And in case of without stent, only aspirin was administered for a month.
Clinical outcomes were assessed with the modified Rankin Scale, which was applied throughout hospitalization and at the last available clinical follow-up. Visual function tests of visual acuity, visual fields, and fundus examination were performed before and after the coil embolization by ophthalmologist during hospitalization.
Immediate angiographic results following coil embolization were evaluated using a 3-point Raymond-Roy classification: class 1; complete obliteration, class 2; residual neck, class 3; residual aneurysm.16) We also confirmed whether anterograde flow of OphA was preserved after coiling.
We used magnetic resonance angiography as the routine follow-up imaging modality for all patients. Follow up imaging studies were usually done at 6, 12 and 24 or 36 months after coil embolization. If recanalization of treated aneurysm was suspected on follow-up MRA, DSA was subsequently performed to further evaluate the patient.
Of the 20 OphA aneurysms in this study, 13 aneurysms (11 with an unruptured aneurysm, 2 with a ruptured aneurysm) in 12 patients underwent the manual carotid compression test during the diagnostic cerebral angiography. The test was not done in 5 patients with ICA type of OphA aneurysms. Two patients of OphA type aneurysms were excluded because of proximal ICA stenosis.
Of the 13 cases underwent the test, 12 cases were able to identify choroidal blush or retrograde blood flow from ECA with manual carotid compression. 8 of positive collaterals were confirmed in first attempt of test, 3 in second test, and 1 in third test. No test-related complication occurred. The characteristics of patients and aneurysms, and results of our study were detailed in Table 1.
A total of 20 endovascular coil embolizations were performed in 19 patients, including 12 with stent-assisted technique, 4 with double catheter technique, and 4 with single catheter technique. Immediate angiographic assessment revealed complete obliteration in 9 aneurysms (45%), residual neck in 9 (45%), and residual aneurysm in 2 (10%).
OphA occlusion occurred in one patient who underwent simple coiling. The presence of ECA collateral flow to OphA in the patient had been confirmed preoperatively through the manual compression test, visual complications did not occur in the patient (Fig. 2). Other patients also had no difference in ophthalmic examinations between before and after the procedure.
During follow-up period, recurrence of aneurysm was occurred in a patient with ruptured OphA aneurysm. The patient who had initially underwent coiling with double catheters technique, received additional stent-assisted coiling.
We usually did follow up imaging studies at 6, 12 and 24 or 36 months after coil embolization except this ruptured patient. In this case, when we performed the coil embolization, tried to maintain the flow to ophthalmic artery. So, we remain the neck of the aneurysm intentionally, then we followed up DSA within 2 months after the first coil embolization.
OphA is the first-branch of the ICA distal to the cavernous sinus and has rich collateral network from the ECA. These functional anastomosis was introduced by Lasjaunias et al. and Berenstein et al in 1983. This anastomosis shows 3 territories which are the orbital region(via the OphA between the internal maxillary artery and internal carotid artery), the petrous-cavernous region(via the inferolateral trunk and meningohypophyseal trunk) and the upper cervical region(via the ascending pharyngeal, the occipital and the vertebral arteries, including the ascending and deep cervical arteries).3)4)
And there are two anastomotic networks which are called deep anastomotic network and superficial anastomotic network. Among the deep anastomotic network, the most important anastomosis is formed between middle meningeal artery and OphA. And superficial anastomotic network includes anastomosis between supraorbital artery and superior temporal artery & dorsal nasal artery and angular artery of facial artery.3)4)
In this study, we confirmed the collateral channel, between ECA and OphA in 12 cases (92.3%) through the manual carotid compression test. This result is similar to previous studies that used the BTO. Kim et al. reported 92.9% of intact collateral circulation from ECA to OphA using BTO,7) Ahn et al. reported that 85.7% of patent collaterals between ECA and OphA were confirmed through BTO.1) Based on these results, we thought that the manual carotid compression test can be used to confirm collateral flow before the treatment of OphA aneurysm. This is first report on ECA collateral to OphA, confirmed by the manual carotid compression test in patients with OphA aneurysms.
BTO has been used to verify collateral flow or tolerance of patients when a parent artery was anticipated to occlude.9) Although performing BTO at the neck of aneurysm would be a more accurate test than manual compression of proximal ICA, a ballooning within intracranial ICA has a potential risk of intracranial vascular injury consequent to subarachnoid hemorrhage or thromboembolism.2)8)14) In addition, BTO is necessary to place the guiding catheter, require systemic heparinization and work in the intracranial artery. For these reasons, several clinicians have reluctant to perform BTO in settings of usual diagnostic cerebral angiography, and sometimes it is performed under general anesthesia just before coil embolization.7) In ruptured OphA aneurysm, BTO has limitations in its use due to the need to systemic heparinization.
In contrast to BTO, the manual carotid compression test is an easy and accustomed maneuver. This test frequently uses to verify collateral circulation from circle of Willis, such as Allcock's test5)6) for evaluating posterior communicating artery. The test as we did is not so different from such method that many clinicians usually do during cerebral angiography. In addition, this method has the advantage that it can be performed immediately without additional process during diagnostic cerebral angiography. Because of such simplicity and wontedness, we think that the manual carotid compression test for OphA aneurysm is suitable as screening test to identify ECA collaterals. And it can be used as an alternative for patients who are difficult to perform BTO.
In this study, the complication related with the manual carotid compression did not occur. However, the manual compression of ICA also has theoretical risk of complications such as dissection of vessels or thromboembolism. Especially for patients with proximal ICA stenosis or atheroma, manual compression the affected area could make ischemic complications.1) Because of that, we did not perform the manual compression test in patients with proximal ICA stenosis. It could be a limitation of manual compression test, but BTO also could increase the risk of complication when perform to patient with atherosclerosis or stenosis of intracranial ICA. OphA aneurysms with ICA type, which have low risk of occlusion during coil embolization, were also not performed the test not to make unnecessary complication and discomfort to patient in our institution.
During the manual compression test, blood flow of ICA might be incompletely blocked, because of the nature of manual compression. We decided that the absence of arterial back-flow into the ICA during the test was the criteria of complete compression. Incomplete compression of ICA can result in false negative finding of the test. By repeating test, we tried to reduce false-negative results. But repeating test can increase the occurrence of complication and give discomfort to patients. For an adequate test to reduce the number of test attempts, in our experience, a position of angiographic catheter in ECA is more important rather than the compression. Although a facial artery can anastomose with OphA, the major source of collateral blood flow from ECA is an internal maxillary artery and its branches. When the first test showed a negative result, we advanced an angiographic catheter to more distal part of ECA, near the proximal internal maxillary artery. We speculate that this action increased the success rate of this test. In our study, positive results were usually confirmed within two times of tests.
If the angiography was performed at the ECA during the test, it is difficult to confirm that the ICA was completely blocked by manual compression. So, we performed angiography at the CCA, instead of ECA, with manual compression of ICA to confirm the complete blockage of ICA. In such a way, contrast medium was frequently flow in the ICA, and the imaging quality of CCA angiography was usually worse to verify the collateral flow of OPHA than performing at the ECA. Therefore, we think that ECA angiography with manual compression of ICA is more efficient than CCA angiography with ICA compression.
One of obstacle for performing the manual carotid compression test is that the location of common carotid artery bifurcation and the pathway of ICA vary from person to person. Lucev et al.12) reported the level of CCA bifurcation from adult cadavers dissection that the superior border of the thyroid cartilage was 50%, at the level of the inferior border of the hyoid bone was 25%, the superior border of the hyoid bone was 12.5%, and at the level of the inferior border of the thyroid cartilage is 12.5%.12) Higher level of CCA bifurcation could be hard to perform the manual compression because mandibular bone interferes with ICA compression. In addition, there is a variety of pathways and relationships between ECA and ICA.10)12) Therefore, it is important to identify the location of the CCA bifurcation and the direction of the ICA through CCA angiography before performing this test.
This study has some limitations. Our study is a retrospective design without randomization, and it included a small numbers of patients.
References
1. Ahn JH, Cho YD, Kang HS, Kim JE, Cho WS, Jung SC, et al. Endovascular treatment of ophthalmic artery aneurysms: assessing balloon test occlusion and preservation of vision in coil embolization. AJNR Am J Neuroradiol. 2014 Nov-Dec;35(11):2146-2152;



2. Andaluz N, Tomsick TA, Keller JT, Zuccarello M. Subdural hemorrhage in the posterior fossa caused by a ruptured cavernous carotid artery aneurysm after a balloon occlusion test. Case report. J Neurosurg. 2006 8;105(2):315-319.

3. Geibprasert S, Pongpech S, Armstrong D, Krings T. Dangerous extracranial-intracranial anastomoses and supply to the cranial nerves: vessels the neurointerventionalist needs to know. AJNR Am J Neuroradiol. 2009 9;30(8):1459-1468;



5. Hongo H, Inoue T, Tamura A, Saito I. Surgical strategy to minimize ischemia during trapping/resection of giant extracranial carotid artery aneurysm stratified by collateral evaluation. Surg Neurol Int. 2017 8:28



6. Kai Y, Morioka M, Yano S, Nakamura H, Makino K, Takeshima H, et al. External Manual Carotid Compression is Effective in Patients with Cavernous Sinus Dural Arteriovenous Fistulae. Interventional Neuroradiology. 2007 3;13 Suppl 1:115-122;


7. Kim B, Jeon P, Kim K, Yang N, Kim S, Kim H, et al. Endovascular treatment of unruptured ophthalmic artery aneurysms: clinical usefulness of the balloon occlusion test in predicting vision outcomes after coil embolization. J Neurointerv Surg. 2016 7;8(7):696-701;


8. Lesley WS, Bieneman BK, Dalsania HJ. Selective Use of the Paraophthalmic Balloon Test Occlusion (BTO) to Identify a False-Negative Subset of the Cervical Carotid BTO. Minim Invasive Neurosurg. 2006 49(1):34-36;



9. Linskey ME, Jungreis CA, Yonas H, Hirsch WL Jr, Sekhar LN, Horton JA, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994 5;15(5):829-843;


10. Lo A, Oehley M, Bartlett A, Adams D, Blyth P, Al-Ali S. Anatomical variations of the common carotid artery bifurcation. ANZ J Surg. 2006 11;76(11):970-972;


11. Louw L. Different ophthalmic artery origins: Embryology and clinical significance. Clin Anat. 2015 7;28(5):576-583;


12. Lucev N, Bobinac D, Maric I, Drescik I. Variations of the great arteries in the carotid triangle. Otolaryngol Head Neck Surg. 2000 4;122(4):590-591;



13. Mathis JM, Barr JD, Jungreis CA, Yonas H, Sekhar LN, Vincent D, et al. Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol. 1995 4;16(4):749-754;


14. Powers WJ. Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol. 1991 3;29(3):231-240;


15. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001 9;32(9):1998-2004;


Fig. 1
A. In routine left ECA angiography, there was no flow to OphA. B. and C. During a manual carotid compression test, retrograde blood flow from ECA to OphA (black arrows) and choroidal blush (white arrows) appeared.
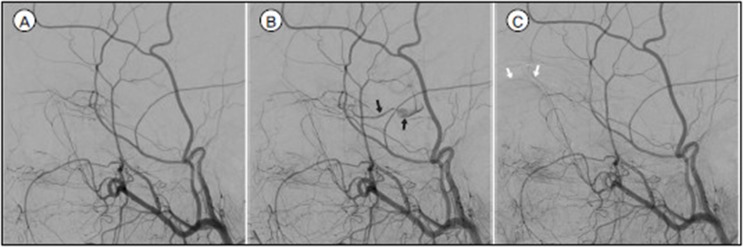
Fig. 2
A. 3-dimensional imaging of DSA of a 65-year-old woman (Case 7) revealed an OphA aneurysm with incorporating the origin of OphA. B. Black arrowhead shows the distal tip of angiographic catheter was located as far away as possible beyond the facial artery. B. and C. Manual carotid compression test confirmed retrograde blood flow from ECA (black arrows) and choroidal blush (white arrows). D. and E. The OphA (white arrowheads) was occluded after coil embolization.
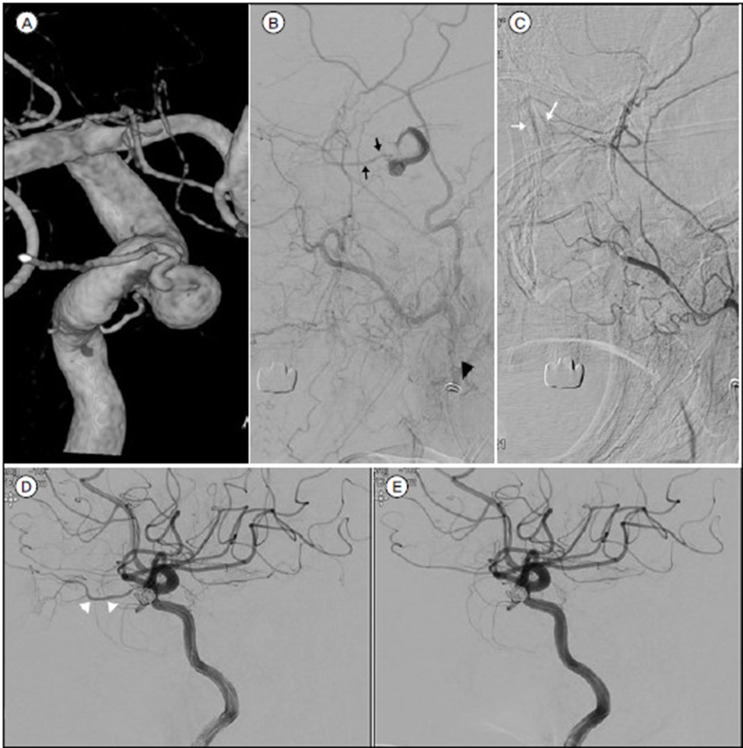
Table 1
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 2,618 View
- 37 Download
- Related articles




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



