 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 16(3); 2014 > Article |
|
Abstract
Objective
In addition to obliterating the aneurysm using clipping or coiling, decompressive surgery for control of rising intracranial pressure (ICP) is thought to be crucial to prevention of adverse outcomes in patients with poor grade aneurysmal subarachnoid hemorrhage (aSAH). We evaluated the clinical characteristics of patients with poor-grade aSAH, and compared outcomes of aneurysmal clipping with simultaneous decompressive surgery to those of coil embolization followed by decompression.
Materials and Methods
In 591 patients with aSAH, 70 patients with H-H grade IV and V underwent decompressive surgery including craniectomy, lobectomy, and hematoma removal. We divided the patients into two groups according to clipping vs. coil embolization (clip group vs. coil group), and analyzed outcomes and mortality.
Results
Aneurysmal clipping was performed in 40 patients and coil embolization was performed in 30 patients. No significant differences in demographics were observed between the two groups. Middle cerebral artery and posterior circulation aneurysms were more frequent in the clip group. Among 70 patients, mortality occurred in 29 patients (41.4%) and 61 patients (87.1%) had a poor score on the Glasgow outcome scale (scores I-III). No significant difference in mortality was observed between the two groups, but a favorable outcome was more frequent in the coil group (p < 0.05).
Conclusion
In this study, despite aggressive surgical and endovascular management for elevated ICP, there were high rates of adverse outcomes and mortality in poor-grade aSAH. Despite poor outcomes overall, early coil embolization followed by decompression surgery could lead to more favorable outcomes in patients with poor-grade aSAH.
Poor-grade aneurysmal subarachnoid hemorrhage (aSAH; Hunt and Hess grade (H-H grade) IV and V) is a significant cause of morbidity and mortality in the general population. Despite recent remarkable advances in microsurgical technique, endovascular coiling, and neurointensive care, patient outcome remains dismal.2)3) These poor outcomes are affected by many factors, including initial injury at the time of aneurysmal rupture, intracranial hypertension due to acute hydrocephalus and intracranial hemorrhage (ICH), increase in cerebrospinal fluid outflow resistance, cerebral edema, or a combination of these factors.21)27) In most cases, recurrent bleeding of a ruptured aneurysm is correlated with significantly mortality.29) Active management protocols for poor-grade aSAH patients have recently been developed and adopted in many neurosurgical centers, such as decompressive surgery, including ventriculostomy, decompressive craniectomy, hematoma removal, and lobectomy.2)28) Several studies supporting the concept that decompressive surgery imparts a benefit for poor-grade aSAH patients have been reported.10)23)30) Therefore, performing decompressive surgery when neurological and radiological signs confirm the presence of increased intracranial pressure (ICP) may reduce mortality and morbidity rates.
In many cases, neurosurgeons perform concomitant decompressive craniectomy and aneurysmal clipping if performance of decompressive surgery is necessary. However, in several clinical studies, early endovascular treatment led to better outcomes in poor-grade aSAH patients due to a reduced chance of rebleeding and reduced treatment delays.5)31) In other words, we assumed that despite the need to perform decompression surgery in a timely fashion, there may be an outcome advantage if endovascular treatment for aneurysm obliteration is administered initially followed by decompressive craniectomy. Therefore, the purpose of this study is to compare the outcomes of poor-grade aSAH patients treated with concomitant decompressive craniectomy and clipping versus coiling followed by decompressive surgery.
Between June 2006 and December 2013, 591 patients were admitted to the neurosurgical intensive care unit due to aSAH. Among them, 70 consecutive patients with H-H grade IV and V who underwent aneurysmal clipping or endovascular coiling for aneurysmal obliteration underwent an additional decompressive surgery for control of ICP, such as hemi- or bilateral craniectomy, lobectomy of brain, and hematoma removal. We retrospectively reviewed patients' hospital records, operative reports, and radiologic findings (three dimensional-computed tomography angiography (3D-CTA) and conventional cerebral angiography (CAG)). Outcomes were assessed on discharge using the Glasgow outcome scale (GOS): grade I (death), grade II (vegetative state), grade III (severe disability), grade IV (moderate disability), and grade V (good recovery). GOS grades I, II, and III were described as unfavorable outcomes, whereas grades IV and V were favorable outcomes.
In our hospital, management of poor-grade aSAH involves an aggressive early intervention policy. Prompt resuscitation, including endotracheal intubation, mechanical ventilation, and mannitol administration, was implemented. Clinical grade was assessed after initial resuscitation, and then 3D-CTA and CAG were performed as soon as possible. If an aneurysm was identified and judged to be the cause of SAH, early treatment to obliterate the ruptured aneurysm (using aneurysmal clipping or endovascular coiling) was administered to reduce the rebleeding rate. In deciding between treatment modalities, we considered patient demographic and radiologic factors: clinical status, operation risk (age, medication, underlying disease), CT findings (brains swelling, accompanying massive ICH or subdural hematoma, or both), and angiographic findings (location and morphology of the aneurysm). We preferred performing decompressive craniectomy and clipping simultaneously if decompression was necessary due to increased ICP. On the other hand, we attempted endovascular treatment in advance of decompressive surgery in cases where aneurysm obliteration could be achieved via the classic single-catheter method without incorporating any branches into the neck of the aneurysm or in cases of wide-necked aneurysm. Endovascular treatment was also attempted when surgical access difficulties were expected due to massive brain swelling with excessive brain retraction and destruction, difficult anatomical characteristics, and an aneurysmal location in the posterior circulation. In this study, we formed two subgroups based on surgical treatment modality: clip group and coil group.
Patients were transferred emergently to the operating room and decompressive surgeries were performed. The decompressive surgeries performed in our institution could be divided into three types, "decompressive craniectomy only", "craniectomy with additional lobectomy", and "craniectomy with ICH removal". Except for patients for whom aneurysm neck clipping had been planned, the head was positioned conventionally. In clip groups, three-point fixation of the head was performed appropriately so that a generous and extended pterional bone flap could be removed. A large, reverse question mark scalp flap was created and fronto-temporo-parietal bone flap was removed in all cases except craniectomies performed for aneurysm neck clipping. Duraplasty was performed similarly in a semi-watertight fashion. When performance of additional lobectomies was required, temporal lobes were resected initially and additional frontal lobectomies were performed in cases when decompressions were thought not to be adequate through temporal lobectomies only. Similarly, ICH was removed via transsylvian route and the transcortical approach was applied only when needed, such as removal of a huge volume of hematomas. Craniectomies were performed in the same setting during performance of aneurysm neck clipping, whereas in the coil group decompressive surgeries were performed only after the coiling embolizations were completed.
To evaluate the differences between the clip group and coil group, the variables were compared using univariate methods (two-sample t-test or chi-square test). MedCalc® (version 13, MedCalc Software bvba, Ostend, Belgium) was used for analysis, and a p value less than 0.05 was considered statistically significant.
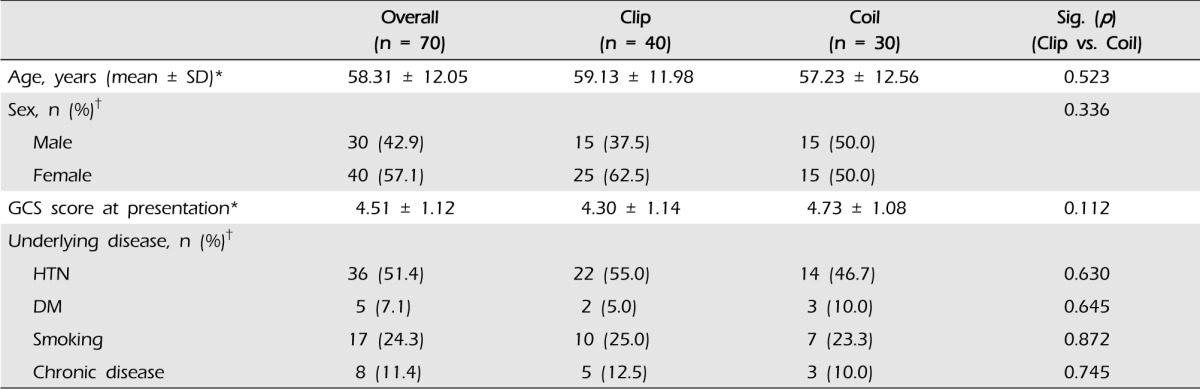
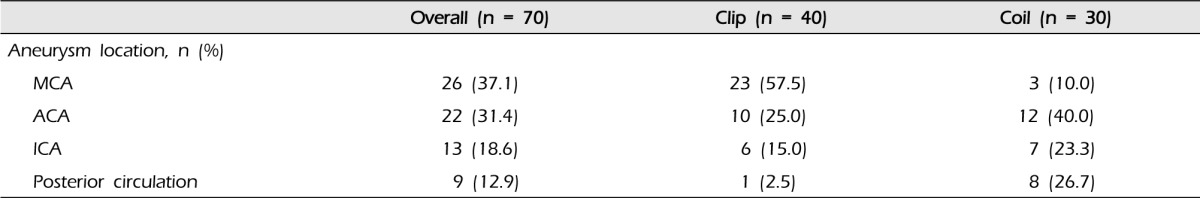
The demographic and clinical characteristics of patients are shown in Table 1. Among 70 patients (mean age: 58.31 ± 12.05 years, male/female: 30/40), 40 underwent aneurysmal clipping (mean age: 59.13 ± 11.98, male/female: 15/25) and 30 underwent endovascular coiling (mean age: 57.23 ± 12.56, male/female: 15/15). At presentation, the mean Glasgow coma scale score (GCS) of patients was 4.51 ± 1.12 (clip group 4.30 ± 1.14, coil group 4.73 ± 1.08; p = 0.112). The most common underlying disease or risk factor was hypertension and the second most common was smoking. However, age, sex, and presenting GCS score did not differ significantly between the two groups. The locations of ruptured aneurysms are shown in Table 2. Middle cerebral artery (MCA, n = 26) aneurysms were most frequent, and anterior cerebral artery (ACA, n = 22) aneurysms were the next most common. In the clip group, 23 of 40 patients had an MCA aneurysm (57.5%) and 10 patients had an ACA aneurysm (25.0%). Twelve of 30 patients in the coil group had an ACA aneurysm (40.0%), eight patients had a posterior circulation aneurysm (26.7%), and seven patients had an internal carotid artery (ICA) aneurysm (23.3%).
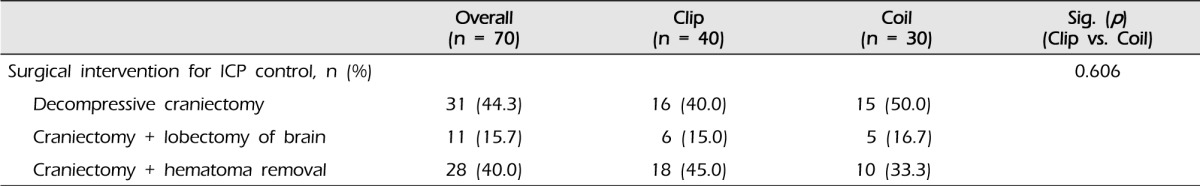
Overall, decompressive craniectomy was performed in 31 of 70 patients. Additional lobectomy and hematoma removal were performed in 11 and 28 patients, respectively. In the clip group, 16 of 40 patients (40.0%) underwent decompressive craniectomy: six patients (15.0%) underwent lobectomy and 18 patients (45.0%) underwent hematoma removal. In the coil group, 15 of 30 patients (50.0%) underwent decompressive craniectomy: five patients (16.7%) underwent lobectomy and 10 patients (33.3%) underwent hematoma removal. No significant difference was observed between the two groups (p = 0.606, Table 3).
At discharge, 61 of 70 patients had an unfavorable outcome (GOS I - III, 87.1%); the remaining 12.9% (28 of 70) were GOS IV or V (favorable outcome). In the clip group (n = 40), 38 (95.0%) patients had an unfavorable outcome, with the two remaining patients having favorable outcomes. By contrast, in the coil group, 23 of 30 patients had GOS I-III (unfavorable outcome, 76.7%), while seven patients had favorable outcomes (23.3%). In comparison of the two groups (clip vs. coil group), the coil group had significantly better outcomes (p = 0.035 and 0.032). However, no significant difference was observed between the mortality rates of the two groups (45.0% in the clip group and 36.7% in the coil group; p = 0.625). In addition, there was no difference in the rate of brain injury causing mortality (p = 0.941, Table 4).
Poor-grade aSAH is a noted cause of morbidity and mortality in the general population. Despite recent advances in microsurgical technique, endovascular coiling and improved neurointensive care units, the outcomes of these patients remain very poor. These outcomes result from the combination of several pathologic mechanisms, including initial brain injury at the time of hemorrhage, delayed brain swelling with intracranial pressure elevation, and vasospasm.21) In addition, elevated ICP due to acute hydrocephalus and focal hematoma in SAH patients leads to poor patient outcomes.6) Fisher and Ojemann described a "vicious feed-forward cycle," which they explained as elevated ICP impairing arterial inflow and venous outflow → ischemic damage to brain causing cerebral edema → swelling → more anoxia.12)
Recently, the role of decompressive craniectomy in the setting of traumatic brain injury, cerebral infarction, and aSAH has been studied at many institutions.9)12) aSAH patients with ICH have worse presentations and more unfavorable outcomes compared to aSAH patients without ICH.2)17)18) The rationale for use of decompressive craniectomy for poor-grade aSAH patients with or without focal ICH are immediate ICP reduction and control of delayed ICP elevation after hemorrhage as a result of delayed brain swelling.15) Many studies supporting the efficacy of decompressive craniectomy have been published.7)8)14)16)30) Rapid and long-lasting control of ICP using decompressive craniectomy has been shown to improve outcomes in stroke, trauma, and aSAH patients.13)20)23)24)25)
In this study, we selected the appropriate intervention for obliteration of the aneurysm primarily according to the angiographic findings of aneurysm rather than age, clinical grade, or predicted prognosis. In this study, no difference in demographic characteristics was observed between the two subgroups (Table 1). However, the location of the ruptured aneurysm differed between groups. In the clip group, MCA aneurysm was most common and ACA was the second most common. By contrast, in the coil group, ACA aneurysm was the most common and posterior circulation aneurysm was the second most common (Table 2). We assumed that these results reflected the combination of many factors, including angiographic findings, morphology of aneurysms, and vascular access.
Natural history- and population-based investigations indicate that only 5% of poor-grade patients without treatment are expected to survive when admitted to the hospital within three days of aSAH.1)4)19) Several authors concluded that early, aggressive treatment and intensive care for patients with poor-grade aSAH can improve mortality and morbidity rates.2) We have abided by an early and aggressive treatment policy for several years, except in patients with unstable vital signs. In this study, the overall mortality rate of poor-grade aSAH patients was 41.4% (clip group, 45.0% vs. coil group, 36.7%), and there was no significant variation between groups (p = 0.625). Favorable outcomes were slightly more frequent in the coil group than in the clip group, and this difference was statistically significant (p = 0.032, Table 4). This difference may be due to the excessive brain retraction causing brain damage or unavoidable removal of brain tissue during microsurgical clipping for poor-grade aSAH. In addition, there was no delay from the time of cerebral angiography to the beginning of endovascular coiling. Because coiling in 26 (86.7%) of 30 coiling patients was performed under local anesthesia using intravenous sedatives, we were able to perform coil embolization just after the diagnostic angiography with no time delay. Considering a mean delay of 127 minutes (range 85-210) from diagnostic angiography to the start of operation under general anesthesia, the time interval from presentation of aSAH to aneurysm obliteration by coil embolization followed by decompressive surgery was shorter than that of concomitant decompressive surgery and clipping. Although we found no statistical evidence of increasing tendency of rebleeding, this is a point worth considering.
According to several recently published studies, many hospitals have adopted an early, aggressive treatment policy for poor-grade aSAH patients.10)23) In addition, many investigations regarding early endovascular coiling followed by decompressive surgery and concomitant decompressive surgery with clipping have been published.11)22)26) Reyes et al.11) reported that endovascular coiling followed by ICH evacuation may be an alternative treatment to concomitant decompressive surgery in patients with both SAH and ICH (mortality rate of treatment: clip, 25% vs. coil, 30%), and has the advantages of short total operation time, short interval from presentation to aneurysm obliteration, and no difference in cost between treatment modalities. Mocco et al.,22) in a prospective study of 148 patients who underwent microsurgery (66%) or received endovascular treatment (34%), reported an overall good outcome rate of 40% and a mortality rate of 43%. Sandsrom et al.,26) who published a retrospective outcome analysis of 123 poor-grade patients treated with either neurosurgical clipping or endovascular coiling, found that 56% had a good outcome and the mortality rate was 22% when utilizing early and aggressive treatment. The treatment results of our study (good outcome rate of 12.9% and mortality rate of 41.5%) are slightly worse than reported in the above mentioned studies.
This study has several limitations, including that it is a single-center investigation with a small cohort and a retrospective study design. In addition, our institutional bias toward early aggressive treatment of poor-grade aSAH resulted in the lack of a delayed treatment group for comparison. However, this study showed the comparative results of two common treatment modalities for poor-grade aSAH. Although the majority of outcomes remained poor, early coil embolization without delay followed by decompression surgery could result in more favorable outcomes in poor-grade aSAH than treatment with clipping.
Despite our early and aggressive management policy for poor-grade aSAH, the results of this study showed unfavorable outcomes. In addition, almost all patients required decompressive surgery with or without lobectomy or hematoma removal. More favorable results were observed for early coil embolization followed by decompressive surgery than for concomitant decompressive surgery and aneurysmal clipping. In conclusion, if angiographic and radiologic finding of aneurysm is suitable to endovascular treatment, consideration of endovascular coiling followed by decompressive surgery before concomitant decompressive craniectomy and clipping may be important.
References
1. Alvord EC Jr, Loeser JD, Bailey WL, Copass MK. Subarachnoid hemorrhage due to ruptured aneurysms. A simple method of estimating prognosis. Arch Neurol. 1972 10;27(4):273-284;


2. Bailes JE, Spetzler RF, Hadley MN, Baldwin HZ. Management morbidity and mortality of poor-grade aneurysm patients. J Neurosurg. 1990 4;72(4):559-566;


3. Baskaya MK, Menendez JA, Yuceer N, Polin RS, Nanda A. Results of surgical treatment of intrasylvian hematomas due to ruptured intracranial aneurysms. Clin Neurol Neurosurg. 2001 4;103(1):23-28;


4. Bonita R, Thomson S. Subarachnoid hemorrhage: epidemiology, diagnosis, management, and outcome. Stroke. 1985 Jul-Aug;16(4):591-594;


5. Bracard S, Lebedinsky A, Anxionnat R, Neto JM, Audibert G, Long Y, et al. Endovascular treatment of Hunt and Hess grade IV and V aneurysms. AJNR Am J Neuroradiol. 2002 Jun-Jul;23(6):953-957;


6. Bundgaard H, Landsfeldt U, Cold GE. Subdural monitoring of ICP during craniotomy: thresholds of cerebral swelling/herniation. Acta Neurochir Suppl. 1998 71:276-278;


7. Burkert W, Paver HD. [Decompressive trepanation in therapy refractory brain edema]. Zentralbl Neurochir. 1988 49(4):318-323; German.

8. Burkert W, Plaumann H. [The value of large pressure-relieving trepanation in treatment of refractory brain edema. Animal experiment studies, initial clinical results]. Zentralbl Neurochir. 1989 50(2):106-108; German.

9. Carter BS, Ogilvy CS, Candia GJ, Rosas HD, Buonanno F. One-year outcome after decompressive surgery for massive nondominant hemispheric infarction. Neurosurgery. 1997 6;40(6):1168-1175; discussion 1175-6.


10. D'Ambrosio AL, Sughrue ME, Yorgason JG, Mocco JD, Kreiter KT, Kreiter KT, Mayer SA, et al. Decompressive hemicraniectomy for poor-grade aneurysmal subarachnoid hemorrhage patients with associated intracerebral hemorrhage: clinical outcome and quality of life assessment. Neurosurgery. 2005 56(1):12-19; discussion 19-20.



11. de los Reyes K, Patel A, Bederson JB, Frontera JA. Management of subarachnoid hemorrhage with intracerebral hematoma: clipping and clot evacuation versus coil embolization followed by clot evacuation. J Neurointerv Surg. 2013 3;5(2):99-103;


12. Fisher CM, Ojemann RG. Bilateral decompressive craniectomy for worsening coma in acute subarachnoid hemorrhage. Observations in support of the procedure. Surg Neurol. 1994 1;41(1):65-74;


13. Forsting M, Reith W, Schabitz WR, Heiland S, von Kummer R, Hacke W, et al. Decompressive craniectomy for cerebral infarction. An experimental study in rats. Stroke. 1995 2;26(2):259-264;


14. Gaab M, Knoblich OE, Fuhrmeister U, Pflughaupt KW, Dietrich K. Comparison of the effects of surgical decompression and resection of local edema in the therapy of experimental brain trauma. Investigation of ICP, EEG and cerebral metabolism in cats. Childs Brain. 1979 5(5):484-498;

15. Gebel JM, Brott TG, Sila CA, Tomsick TA, Jauch E, Salisbury S, et al. Decreased perihematomal edema in thrombolysis-related intracerebral hemorrhage compared with spontaneous intracerebral hemorrhage. Stroke. 2000 3;31(3):596-600;


16. Guerra WK, Gaab MR, Dietz H, Mueller JU, Piek J, Fritsch MJ. Surgical decompression for traumatic brain swelling: indications and results. J Neurosurg. 1999 2;90(2):187-196;


17. Hauerberg J, Eskesen V, Rosenorn J. The prognostic significance of intracerebral haematoma as shown on CT scanning after aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 1994 8(3):333-339;


18. Hayashi M, Marukawa S, Fujii H, Kitano T, Kobayashi H, Yamamoto S. Intracranial hypertension in patients with ruptured intracranial aneurysm. J Neurosurg. 1977 5;46(5):584-590;


19. Hijdra A, Braakman R, van Gijn J, Vermeulen M, van Crevel H. Aneurysmal subarachnoid hemorrhage. Complications and outcome in a hospital population. Stroke. 1987 Nov-Dec;18(6):1061-1067;


20. Kalia KK, Yonas H. An aggressive approach to massive middle cerebral artery infarction. Arch Neurol. 1993 12;50(12):1293-1297;


21. Milhorat TH. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1987 1;20(1):15-20;


22. Mocco J, Ransom ER, Komotar RJ, Schmidt JM, Sciacca RR, Mayer SA, et al. Preoperative prediction of long-term outcome in poor-grade aneurysmal subarachnoid hemorrhage. Neurosurgery. 2006 9;59(3):529-538; discussion 529-38.



23. Otani N, Takasato Y, Masaoka H, Hayakawa T, Yoshino Y, Yatsushige H, et al. Surgical outcome following decompressive craniectomy for poor-grade aneurysmal subarachnoid hemorrhage in patients with associated massive intracerebral or Sylvian hematomas. Cerebrovasc Dis. 2008 26(6):612-617;


24. Rieke K, Schwab S, Krieger D, von Kummer R, Aschoff A, Schuchardt V, et al. Decompressive surgery in space-occupying hemispheric infarction: results of an open, prospective trial. Crit Care Med. 1995 9;23(9):1576-1587;


25. Rinaldi A, Mangiola A, Anile C, Maira G, Amante P, Ferraresi A. Hemodynamic effects of decompressive craniectomy in cold induced brain edema. Acta Neurochir Suppl (Wien). 1990 51:394-396;

26. Sandstrom N, Yan B, Dowling R, Laidlaw J, Mitchell P. Comparison of microsurgery and endovascular treatment on clinical outcome following poor-grade subarachnoid hemorrhage. J Clin Neurosci. 2013 9;20(9):1213-1218;


27. Sarrafzadeh A, Haux D, Kuchler I, Lanksch WR, Unterberg AW. Poor-grade aneurysmal subarachnoid hemorrhage: relationship of cerebral metabolism to outcome. J Neurosurg. 2004 3;100(3):400-406;

28. Shin YS, Kim SY, Kim SH, Ahn YH, Yoon SH, Cho KH, et al. One-stage embolization in patients with acutely ruptured poor-grade aneurysm. Surg Neurol. 2005 2;63(2):149-154; discussion 154-5.


29. Sluzewski M, van Rooij WJ. Early rebleeding after coiling of ruptured cerebral aneurysms: incidence, morbidity, and risk factors. AJNR Am J Neuroradiol. 2005 8;26(7):1739-1743;


Table 1
Demographic characteristics of patients with poor-grade aneurysmal subarachnoid hemorrhage who underwent aneurysm obliteration and decompressive surgery*
- TOOLS
-
METRICS

-
- 25 Crossref
- 0 Scopus
- 2,564 View
- 37 Download
- Related articles





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



