INTRODUCTION
Thyroid hormones directly affect endothelial and vascular smooth muscle cells, and high levels of thyroid hormones are known to cause hypertrophy and stiffness of the vessels.12)20) Some authors have reported that coronary vasospasms occur as a result of hyperthyroidism accompanied by myocardial infarction.11)16)22) However, the reverse causal relationship has rarely been reported. Furthermore, cerebral vasospasm (CV) associated with hyperthyroidism has never been reported to cause cerebral infarction. We therefore report here a rare and first of its kind case of severe CV that was caused by cerebral infarction in a patient with mild head injury and hyperthyroidism.
CASE REPORT
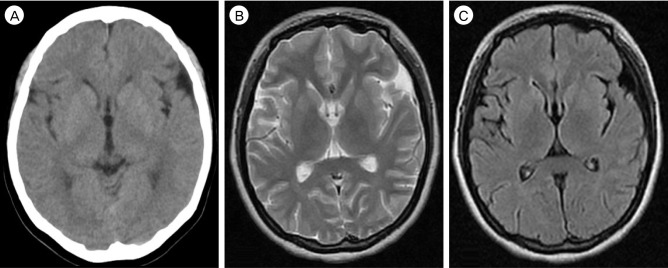
A 30-year-old woman was transferred to our hospital in a stuporous state with right hemiparesis. She had earlier been admitted to another hospital for two weeks after she was injured in a traffic accident. On admission to our hospital, she first complained of headache and dizziness only. She was alert and had no neurological deficits. Initial brain computed tomography (CT) and magnetic resonance (MR) images taken at the other hospital showed no abnormal lesions in the intracranial space (Fig. 1). She was diagnosed with hyperthyroidism 2 months ago, and has been taking 15 mg of methimazole and 40 mg of propranolol daily. She had no past history of other diseases. On admission, laboratory investigations showed low levels of thyroid stimulating hormone (TSH) (0.011 ┬ĄIU/mL, reference range: 0.27-4.2 ┬ĄIU/mL), low levels of free T4 (0.644 ng/dL, reference range: 0.93-1.7 ng/dL), elevated levels of triiodothyronine (T3) (2.58 ng/mL, reference range: 0.8-2.0 ng/mL) and elevated levels of thyrotropin-binding inhibitory immunoglobulin (TBII) (62.80 IU/L, reference range: 0-15 IU/L). MR angiography demonstrated cerebral infarction in the posterior cerebral artery (PCA) territories and multiple stenotic lesions in both middle cerebral arteries (MCAs), anterior cerebral arteries (ACAs), and PCAs (Fig. 2A). Cerebral angiography also revealed severe vasospasms in all the MCA, ACA and PCA territories (Fig. 2B-D). Therefore, intra-arterial chemical angioplasty with verapamil was performed for two consecutive days. A total of 25 mg of verapamil was injected slowly: 10 mg each for the left and right internal carotid artery and 5 mg for the left vertebral artery. Successful dilatation of the spastic arteries was demonstrated (Fig. 2E-G). However, cerebral angiography performed the next day still showed some remnant vasospasm. Thus, chemical angioplasty was performed again using 15 mg of verapamil. After the procedures, the patient was given antithyroid medication and "triple-H" therapy (hypervolemia, hypertension, and hemodilution) to increase cerebral perfusion. Follow-up angiography performed at 6 weeks demonstrated complete recovery of the vasospasm (Fig. 3). At the 2-year clinical follow up, she was alert with mild weakness and cortical blindness (modified Rankin scale score, 4).
DISCUSSION
CV is common in patients with aneurysmal subarachnoid hemorrhage (SAH), and it is a leading cause of morbidity and mortality.8) CV in traumatic SAH is characterized by earlier onset and milder clinical symptoms than that in aneurysmal SAH.10) Post-traumatic vasospasm without traumatic SAH has a brief duration of moderate spasms with an average time course of 1.25 days.15) However, this is the first report on CV in a patient with mild head injury and hyperthyroidism.
In our case, CV could not be concluded from a simple mild head trauma without hemorrhage, so other risk factors associated with CV needed to be investigated. Hyperthyroidism was confirmed 2 months ago, and there was no other medical history except for elevation of T3 and TBII. Therefore, we focused on the relationship between CV and hyperthyroidism.
The association between CV and hyperthyroidism has not been proven clearly yet. Cerebrovascular changes in hyperthyroidism have been reported to be limited to Moyamoya disease (MMD). A recent study of patients with MMD reported a high incidence of elevated thyroid function and thyroid autoantibody levels.2)13) Thyrotoxicosis may be the trigger of the vascular attack in MMD patients that results in the increase in sympathetic nervous system sensitivity.9) Choi et al. have reported about coronary vasospasms with thyrotoxicosis in 6,923 subjects; they showed that the hyperthyroid state enhances the sensitivity of the vascular contractile responses to either norepinephrine (NE) or 5-hydroxytryptamine (5-HT). Catecholamines can induce vasospasm in patients with coronary vasospasm.3)
In general, many studies have shown that the thyroid hormone is an important regulator of endothelial nitric oxide (NO) production and vascular tone.7) Moreover, hyperthyroidism was shown to enhance the sensitivity of resistant vessels to the vasoconstrictive action of NE.17) Cerebral vascular reactivity is not very different from typical vascular reactivity. Cerebral arteries are innervated by noradrenaline (NA)- and 5-HT-containing nerves,14) and NE is known to play a role in inducing a dose-mediated vasoconstrictive effect on cerebral vasculature.5)23) We therefore hypothesize that vasoconstriction may be exacerbated by hyperthyroidism and that stressful situations may result in NE secretion and then cerebral infarction after CV.
There are certain limitations to our study. We have reported only one case for which there is no clear evidence of the association between hyperthyroidism and CV. Nonetheless, it seems that mild head trauma was induced by a stressful event in her condition, and the absence of medication in the hyperthyroid state enhanced the sensitivity of the cerebral vascular contractile responses. In this situation, cerebral ischemia occurred because of CV.
In general, management of traumatic brain injury-related CV can be quite different from treatment of aneurysmal SAH-related CV because of the severity of the injury and associated comorbidities.10) However, in our case, hemorrhage did not occur, and triple-H therapy and endovascular chemical angioplasty were performed at the same time as in cases of aneurysmal SAH-related CV. Endovascular therapy for CV has a safety efficacy profile that shows neurological improvement.1)6) Papaverine, verapamil, nimodipine and nicardipine have been used as intra-arterial vasodilators to treat patients.21) Therefore, intra-arterial verapamil was administered in our case.
Previously published cases about coronary vasospasm and MMD with hyperthyroidism demonstrated that restoration of euthyroidism was a priority.4)18)19) According to Ni et al., a sudden increase in the thyroid hormone levels should be avoided to prevent cerebrovascular accidents, because cerebrovascular hemodynamic changes due to thyrotoxicosis might be responsible for CV.18) The treatment of hyperthyroidism was accompanied by neurological improvement. In the case of our patient, we administered medication for hyperthyroidism at the endocrinology department.
CONCLUSION
Hyperthyroidism may influence cerebral vascular hemodynamics. Therefore, a sudden increase in thyroid hormone levels in the clinical setting should be avoided to prevent cerebrovascular accidents. When neurological deterioration is noticed without primary cerebral parenchymal lesions, an evaluation of thyroid function may be required before cerebrovascular symptoms occur.






 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print