 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 18(1); 2016 > Article |
|
Abstract
A man visited the emergency room with a headache. Brain computed tomography showed aneurysmal subarachnoid hemorrhage (SAH) and multiple aneurysms. After aneurysm clipping surgery, the patient was discharged. After 5 days, he was admitted to the hospital with skin ulceration and was diagnosed with Behcet syndrome. An angiogram taken 7 weeks after aneurysmal SAH showed intracranial vasospasm. Because inflammation in Behcet syndrome may aggravate intracranial vasospasm, intracranial vasospasm after aneurysmal SAH in Behcet syndrome should be monitored for longer compared to general aneurysmal SAH.
Behcet syndrome is a chronic autoimmune vasculitis with a possible genetic predisposition.4) Recurrent genital and oral ulcerations, erythema nodosum, and uveitis are conspicuous findings. Vasculitis may involve both small and large vessels of all types, to varying degrees.4) In particular, arterial involvement of Behcet syndrome is identified as stenosis, aneurysm, or thrombosis.1)4) Aneurysm due to Behcet syndrome is dangerous and may cause life-threatening outcomes.19) We report a case of Behcet syndrome with multiple cerebral aneurysms and delayed vasospasm for 2 months after aneurysmal subarachnoid hemorrhage (SAH). In this study, we adhered to the Declaration of Helsinki guidelines for studies with humans.
A 45-year-old man visited the emergency room with a 2-week history of headache and left side facial palsy. He had no medical history. Magnetic resonance imaging (MRI) and angiographic computed tomography showed aneurysmal SAH and multiple intracranial aneurysms at the middle cerebral artery (MCA) bifurcation and anterior communicating artery (ACoA). Digital subtraction angiography (DSA) revealed severe vasospasm on the A1 segment of the anterior cerebral artery (ACA) and on both sides of the M1 segment of the MCA (Fig. 1A, B). The patient underwent for clipping of the multiple intracranial aneurysms. A ruptured daughter sac in the ACoA was observed in the surgical field. The bone flap was left uncovered to allow for brain swelling. After surgery, endovascular chemical angioplasty, using nimodipine (2-5 mg), and balloon angioplasty was performed for 3 days. Blood flow to the distal segment of the intracranial artery improved. His clinical symptoms also improved gradually. Before the patient was discharged from the hospital, DSA showed increased blood flow to the distal segment of the intracranial artery (Fig. 1C, D). The patient was discharged symptom-free on the 18th day of hospitalization.
After 5 days, the patient visited the outpatient clinic with odynophasia. Physical examination revealed oral aphthous and genital ulcerations. The patient was admitted to the hospital for treatment of odynophasia and the dermatologic symptoms. We consulted the dermatologic department regarding the ulcerations. The result of the pathergy skin test was positive; therefore, the patient received pulse steroid therapy for 2 weeks under the diagnosis of Behcet syndrome. Pulse methylprednisolone was initiated (1,000 mg/day for 3 days; prednisolone, 60 mg/day for 3 days), followed by daily oral prednisolone at 1 mg/kg/day for 7 days. The oral aphthous and genital ulcerations improved gradually.
However, the patient abruptly developed Grade 1 right arm weakness on day 16 of the second admission, 7 weeks after the aneurysmal SAH. Emergency DSA showed severe vasospasm in the A1 and A2 segments of the ACA on both sides, and the M1 segment of the MCA on the left side (Fig. 2A and 2B), with reduced flow in the distal vessels. During DSA, the narrowed vessels were infused with nimodipine (5 mg) for 3 days. However, on the third day, the left M1 segment of the MCA on the left side was abruptly obstructed (Fig. 2C, D). Although chemical angioplasty using nimodipine (7 mg) and papaverine (30 mg) was performed immediately, the M1 segment of the left MCA was repeatedly occluded. To prevent infarction of the left cerebral hemisphere, the patient underwent an emergency superficial temporal artery (STA) to MCA anastomosis. The distal MCA did not have any pulse and collapsed in the surgical field. Although the previous clip on the left MCA was declamped under-suspicion of clip malposition, distal flow did not improve substantially. After bypass surgery, DSA showed good patency of the STA-MCA anastomosis and improvement of the flow in the MCA (Fig. 2E, F). Right arm weakness also improved (Grade 3) gradually after the operation. The patient was transferred to the Department of Rehabilitation with mild weakness.
This case was characterized by intracranial multiple aneurysms and intracranial delayed vasospasm. Although some researchers have reported intracranial multiple aneurysms in Behcet syndrome,3)6)8) there are no reports of intracranial delayed vasospasm after aneurysmal SAH for 2 months in Behcet syndrome. Indeed, to our knowledge, this is the first report of intracranial delayed vasospasm 2 months after aneurysmal SAH in Behcet syndrome.
Intracranial vasospasm-ischemia associated with aneurysmal SAH has a biphasic course.17) The acute phase is a period of reduction of blood flow after immediate bleeding.17) The delayed phase begins 2-4 days after aneurysmal SAH, and lasts for up to 14 days.17) Several chemical meterials have been implicated in intracranial delayed vasospasm after aneurysmal SAH.7)12)13)17) Oxyhemoglobin, endothelin-1, and prostaglandins have all been described as important vasoconstrictors in aneurysmal SAH.7)12)13) In particular, endothelin-1 has the ability to produce long-lasting vasoconstriction.2)11)22) These chemicals lead to serial reactions producing a tonic state of the vessel wall.
The main pathogenesis of Behcet syndrome is vascular inflammation and angiogenesis.5) This pathogenesis is very similar to serial intracranial delayed vasospasm after aneurysmal SAH because spasmogens, such as endothelin-1 and nitric oxide (NO), in intracranial delayed vasospasm after aneurysmal SAH are analogous to chemical constituents in Behcet syndrome.
Endothelin-1 is increased in active Behcet syndrome20)21) and its secretion from inflammatory vessels may play an important role in the progression of vasculitis.20) Furthermore, endothelin-1 is implicated in the pathogenesis of cerebral vasospasm and delayed cerebral ischemia as a potent vasoconstrictor.18) In addition, endothelin-1 is a potent, long-lasting endogenous vasoconstrictor that has been associated with cerebral vasospasm.2)11)22) Prolonged elevations of endothelin-1 levels caused by Behcet syndrome may cause unusual prolonged vasospasm.16) As a result, this triphasic pattern of delayed vasospasm may be caused by continuously secreted endothelin-1.
NO is also known for producing important constituents in Behcet syndrome.14) NO, an endothelium-derived relaxing factor, is a crucial mediator of the inflammatory process. The concentration of NO is increased in patients with Behcet syndrome and is related with disease activity.10) Furthermore, NO also plays an important role in the regulation of vascular hemodynamic activity.15) It normally dilates vessels by blocking intracellular calcium release from the sarcoplasmic reticulum in smooth muscle cells. However, it shows characteristic alterations after SAH. These changes may affect the auto-regulation of homeostasis and may be related to the pathogenesis of intracranial delayed vasospasm.15) Consequently, increased NO levels and NO functional alteration may cause unusual prolonged vasospasm.
We attempted to confirm an association between levels of specific constituents and intracranial delayed vasospasm. We were unable to perform a blood test because of a lack of sample of Behcet disease; therefore, we attempted to confirm vessel wall inflammation through vessel wall MRI using an Achieva 3.0-T scanner with a 32-channel head coil (Philips, Amsterdam, Netherland). The vessel wall MRI protocol included T1-weighted vessel wall images and proton density images (3-dimensional fast spin echo acquisition with a reconstructed 640 × 640 matrix; slice thickness 0.5 mm; total slap thickness 3.5 cm), which is an emerging technique for characterizing intracranial arterial disease. However, we could not confirm inflammation or wall thickening of the vessels on vessel wall MRI. One researcher has reported that contrast-enhanced MRI is useful for detecting the distribution of vessel wall inflammation, but has less utility in assessing disease activity in Takayasu arteritis.9) We believe that contrast-enhanced MRI in our patient might not have shown wall enhancement because of low disease activity and steroid pulse therapy. We presume that specific chemical constituents might be continuously secreted after the first discharge of the patient. Because of the constant secretion of vasoconstrictor from inflammatory vessels, intracranial delayed vasospasm was more prolonged than expected.
Inflammatory vessels in Behcet syndrome may aggravate intracranial delayed vasospasm after aneurysmal SAH. Therefore, intracranial delayed vasospasm after aneurysmal SAH in Behcet syndrome should be monitored for a longer time compared to general aneurysmal SAH. Such considerations could prevent poor prognosis and death among patients with Behcet syndrome.
NOTES
References
1. Aktas EG, Kaplan M, Ozveren MF. Basilar artery aneurysm associated with Behçet's Disease: a case report. Turk Neurosurg. 2008 1;18(1):35-38;

2. Asano T, Ikegaki I, Suzuki Y, Satoh S, Shibuya M. Endothelin and the production of cerebral vasospasm in dogs. Biochem Biophys Res Commun. 1989 3;159(3):1345-1351;


3. Buge A, Vincent D, Rancurel G, Dechy H, Dorra M, Betourne C. Behçet's diseases with multiple intracranial arterial aneurysms. Rev Neurol (Paris). 1987 12;143(12):832-835;

4. Cevik C, Otahbachi M, Nugent K, Jenkins LA. Coronary artery aneurysms in Behçet's disease. Cardiovasc Revasc Med. 2009 Apr-Jun;10(2):128-129;


5. Choe JY, Park SH, Kim SK. Serum angiopoietin-1 level is increased in patients with Behçet's disease. Joint Bone Spine. 2010 7;77(4):340-344;


6. el Abbadi N, el Mostarchid B, Ababou A, Mosadik A, Semlali A, Bellakhdar F. Behcet's disease with multiple intracranial arterial aneurysms. Report of a case. J Mal Vasc. 1999 6;24(3):225-228;

7. Juvela S. Plasma endothelin and big endothelin concentrations and serum endothelin-converting enzyme activity following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002 12;97(6):1287-1293;


8. Kaku Y, Hamada JI, Kuroda JI, Kai Y, Morioka M, Kuratsu JI. Multiple peripheral middle cerebral artery aneurysms associated with behcet's disease. Acta Neurochir (Wien). 2007 8;149(8):823-827; discussion 827



9. Kato Y, Terashima M, Ohigashi H, Tezuka D, Ashikaga T, Hirao K, et al. Vessel wall inflammation of takayasu arteritis detected by contrast-enhanced magnetic resonance imaging: Association with disease distribution and activity. PLoS One. 2015 12;10(12):e0145855



10. Kiraz S, Ertenli I, Calguneri M, Ozturk MA, Haznedaroglu IC, Altun B, et al. Interactions of nitric oxide and superoxide dismutase in Behçet's disease. Clin Exp Rheumatol. 2001 Sep-Oct;19(5 Suppl 24):S25-S29;
11. Kobayashi H, Ide H, Ishii H, Kabuto M, Handa Y, Kubota T. Endothelin-1 levels in plasma and cerebrospinal fluid-following subarachnoid haemorrhage. J Clin Neurosci. 1995 7;2(3):252-256;


12. Kolias AG, Sen J, Belli A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: Putative mechanisms and novel approaches. J Neurosci Res. 2009 1;87(1):1-11;


13. Macdonald RL. Endothelin antagonists in subarachnoid hemorrhage: What next? Crit Care. 2012 11;16(6):171



14. Murat A, Cemile K, Sema U, Yüksel T, Ramazan Y, Ferah A, et al. Serum nitric oxide, asymmetric dimethylarginine, and plasma homocysteine levels in active Behçets disease. Turk J Med Sci. 012 9;42(Suppl 1):1194-1199.
15. Ng WH, Moochhala S, Yeo TT, Ong PL, Ng PY. Nitric oxide and subarachnoid hemorrhage: Elevated level in cerebrospinal fluid and their implications. Neurosurgery. 2001 9;49(3):622-626; discussion 626-7


16. Nishizawa S, Chen D, Yokoyama T, Yokota N, Otha S. Endothelin-1 initiates the development of vasospasm after subarachnoid haemorrhage through protein kinase c activation, but does not contribute to prolonged vasospasm. Acta Neurochir (Wien). 2000 12;142(12):1409-1415;



17. Nishizawa S, Laher I. Signaling mechanisms in cerebral vasospasm. Trends Cardiovasc Med. 2005 1;15(1):24-34;


18. Thampatty BP, Sherwood PR, Gallek MJ, Crago EA, Ren D, Hricik AJ, et al. Role of endothelin-1 in human aneurysmal subarachnoid hemorrhage: Associations with vasospasm and delayed cerebral ischemia. Neurocrit Care. 2011 8;15(1):19-27;




19. Tsuji S, Suzuki Y, Tomii M, Matsuoka Y, Kishimoto H, Irimajiri S. Behçet's disease associated with multiple cerebral aneurysms and downhill esophageal varices caused by superior vena cava obstruction: a case report. Ryumachi. 1990 10;30(5):375-379; discussion 379-81

20. Ural AU. Increased plasma endothelin-1 levels in active behcet's disease. Clin Rheumatol. 1997 11;16(6):640



Fig. 1
(A, B) Digital subtraction angiography (DSA) showing vasospasms in both the middle cerebral artery (MCA) and the anterior cerebral artery (ACA) on admission. Note the intracranial multiple aneurysms in both the MCA and the anterior communicating artery). (C, D) DSA showing resolved vasospasms in the MCA (arrow) and ACA before discharge.
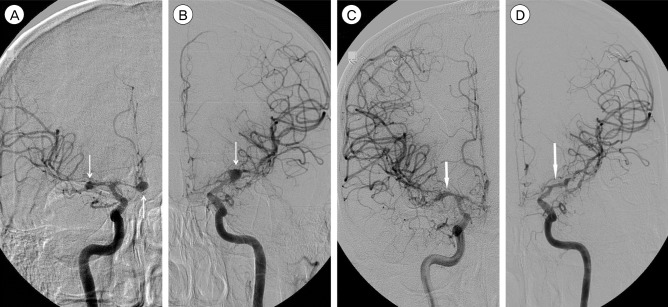
Fig. 2
(A, B) Digital subtraction angiography (DSA) performed in the 7th week after subarachnoid hemorrhage shows vasospasms in both the middle cerebral artery (MCA) and the anterior cerebral artery (ACA) and the disappearance of multiple intracranial aneurysms. (C, D) DSA showing total occlusion of the left MCA. (E, F) DSA showing patent blood flow after left MCA-superficial temporal artery (STA) anastomosis.

- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,260 View
- 23 Download
- Related articles
-
Initial severity of aneurysmal subarachnoid hemorrhage (SAH): Trend over time2022 December;24(4)
Prognosis of Aneurysmal Subarachnoid Hemorrhage in the Elderly Patients.2000 March;2(1)
General Complications of Aneurysmal Subarachnoid Hemorrhage.2001 March;3(1)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



