 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 17(3); 2015 > Article |
|
Abstract
Cerebral cavernous malformations (CMs) are vascular malformations of the central nervous system, which can be detected in the absence of any clinical symptoms. Nodules and cysts with mixed signal intensity and a peripheral hemosiderin rim are considered brain magnetic resonance imaging (MRI) findings typical of CMs. A 48-year-old man was admitted to our hospital because of abnormal MRI findings without significant neurological symptoms. A cyst with an internal fluid-fluid level was found in the left basal ganglia on the initial brain MRI. We decided to observe the natural course of the asymptomatic lesion with serial MRI follow-up. On MRI at the 5-month follow-up, the cystic mass was enlarged and showed findings consistent with those of cystic CM. Surgical resection was performed and the pathological diagnosis was CM. Our experience suggests that the initial presentation of a CM can be a pure cyst and neurosurgeons should consider the likelihood of CMs in cases of cystic cerebral lesions with intracystic hemorrhage.
Vascular malformations of the central nervous system are classified according to four groups: arteriovenous malformation, capillary telangiectasia, cavernous malformation, and venous malformation. Cavernous malformations (CMs) account for 5-15% of all vascular malformations and occur in 0.4-0.8% of the general population.2)9)19) The most common location of cerebral CMs is the supratentorial region, and CMs are occasionally found in the brain stem, basal ganglia, and posterior fossa.4)11) The clinical course of CMs arising from the supratentorial region is mostly benign, however previous studies have shown that recurrent hemorrhage from brainstem CMs can be fatal in up to 17% of patients.15)18) Common symptoms of CMs are headaches, seizures, and focal neurological deficits.1)4)11) CMs arising in the central nervous system usually show typical magnetic resonance imaging (MRI) findings of nodules and cysts with mixed signal intensity and a peripheral hemosiderin rim.11)24) These characteristics are thought to be associated with hemorrhage from the CMs. We report on an unusual radiologic presentation of cystic cerebral CM.
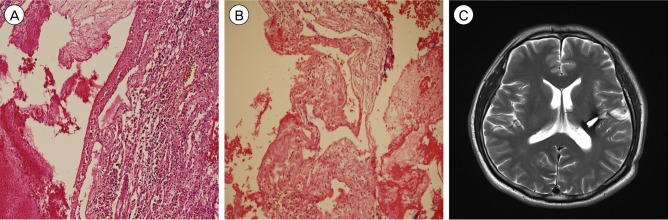
A 48-year-old male was referred to our hospital due to abnormal brain MRI findings. He had undergone brain MRI for a mild headache without other neurological deficits such as weakness, paresthesia, or aphasia. The patient had no family history of intracranial lesions such as stroke, vascular malformations, or tumors. The initial brain MRI and computed tomography (CT) scan showed a single cystic mass located in the left basal ganglia with a fluid-fluid level and without peri-lesional edema (Fig. 1A-C). Because the initial brain MRI was performed without enhancement at another hospital, an enhancing nodular lesion or peripheral rim enhancement could not be verified, however no nodular lesion or capsule-like appearance was detected using T2-, T1-weighted MRI. There was no evidence of calcification of the lesion in CT and gradient recalled echo T2-weighted imaging. To rule out vascular diseases, cerebral angiography was performed, which did not demonstrate tumor staining or a vascular malformation (Fig. 1D). The first repeat brain MRI with Gadolinium enhancement performed 5 months after the initial brain MRI showed no evidence of peripheral or nodular enhancement. A spherical mass with a diameter of 16 mm had enlarged to 25 mm. Suspicious findings consistent with cavernous malformation were observed on axial T2-weighted MRI, which showed a reticulated "salt and pepper" pattern in the core and a peripheral halo of low signal intensity suggesting a hemosiderin rim (Fig. 2A, B). These findings supported a diagnosis of cavernous malformation. The patient's symptoms were not remarkable; therefore, we decided to observe the natural course of the lesion using serial MRI. After 2 months, the patient attended our emergency department with a significant right hemiparesis of 3-hour duration. He was able to move the limbs on his right side through a full range of motion against resistance, but their power was weak. Brain MRI performed immediately showed an increase in the size of the lesion to 35 mm in diameter with heterogeneous internal signal intensity, multi-stage hemorrhage, and aggravated peri-lesional edema (Fig. 2C, D). Based on the imaging findings, the lesion was diagnosed as a CM with recurrent bleeding. A left pterional craniotomy was performed, followed by a trans-sylvian approach, trans-insular cortisectomy for removal of the mass. The margin of the mass was clearly defined and the bulk of the mass had the gross appearance of a hematoma with a yellowish cystic fluid and no encapsulation. A gliotic plane surrounding the mass was observed. There was no active bleeding or evidence of abnormal vessels in the operative field. Intraoperative neuromonitoring included somatosensory evoked potentials (SSEP) and motor evoked potentials (MEP). Median and tibial nerves SSEPs on both sides showed normal values in latency at baseline and no significant interval changes during surgery. MEP decreased by nearly 50% in amplitude at the right extensor digiti minimi during resection of the tumor. Final MEP waveforms after skin closure showed improvement and decreased by < 50% in amplitude and increased < 10% in latency. A neurologic examination performed immediately after surgery showed no significant motor changes compared with the pre-operative examination. After the operation, the patient recovered rapidly from hemiparesis within 2 weeks and was discharged without neurological deficits. The histopathological features were consistent with CM (Fig. 3A, B). After an additional 5 months, follow-up MRI showed no residual lesion (Fig. 3C) and the patient's neurologic status was normal.
Zabramski et al.24) classified cavernous malformations according to four types based on the MRI findings. Type I lesions are characterized by the presence of subacute hemorrhage and show a hyperintense core on T1-weighted images and a hyper- or hypointense core with a surrounding hypointense rim on T2-weighted images. Type II lesions are characterized by a loculated multi-stage hemorrhage surrounded by gliosis and a hemosiderin rim and show a core with reticulated mixed signals on T1-weighted images, and a reticulated mixed-signal core with a surrounding hypointense rim on T2-weighted images. Type III lesions are characterized pathologically by a chronic resolved hemorrhage with hemosiderin staining within and around the lesion. These lesions show a markedly hypointense rim lesion on T2-weighted and gradient-echo images and are iso- or hypointense on T1-weighted images. Type IV lesions are poorly seen or not visualized at all on T1-, T2-weighted images. In Zabramski's study, cystic CMs were not specifically described, but hyperintense lesions on spin-echo MR images are lesions with evidence of subacute or mixed-stage hemorrhage, therefore Type I or II lesions can be regarded as cystic CMs on MRI.
Although the pathophysiological mechanisms for cystic degeneration of CMs are not clear, they are thought to involve recurrent bleeding. The annualized bleeding risk for the first event was reported as 0.4-0.7% and the risk of repeat bleeding was reported as 3.8-29.5%.1)3)8)10)17)18) The accepted mechanism has been that recurrent bleeding occurs from sinusoids or neocapillaries within the CM, leading to changes in osmotic pressure across membranes and causing the gradual accumulation of fluid within the CM.5)6)11)20) These events may cause enlargement of the cystic cavity and MRI findings for CMs can show multi-stage hemorrhage with cystic cavity and a hemosiderin rim. The radiographic features of cystic CMs have been described in various case reports.5)6)7)11)21)23)
In a review of 25 cases of cystic CMs by Ohba et al.,11) there was enhancement of the solid components or the cyst walls in one third to half of the cases. They described the typical MRI findings of cystic CMs as a mixed signal intensity nodule, an iso-to-high and iso-signal intensity cyst on T1- and T2-weighted images, with peripheral hemosiderin deposition at the rim. Based on the CT findings, almost all cystic portions of cystic CMs were low-density in various previous reports.7)11)21)23) In our case, a cystic cavity with a fluid-fluid level was the only observation and no peripheral low-signal-intensity walls, mixed signal intensities, or nodules were observed on the first brain MRI. Calcifications or multiple lesions have also been reported in some cases of cystic CMs,7)16)21)22)23) however there were no such findings in our case.
A satisfactory diagnosis for our patient was not confirmed until a repeat brain MRI had been performed. Differential diagnoses for cystic CMs may include tumors such as gliomas, metastatic tumors, or hemangioblastomas, and other non-neoplastic conditions such as abscesses or parasitic cysts.11)23) However, because no evidence of a solid component was found on CT and MRI, such diagnoses were not appropriate in our case. A neuroglial cyst was also considered as a differential diagnosis because our case initially presented as a pure intra-parenchymal cyst. However, the cystic fluid showed signal intensities that differed from the typical findings of neuroglial cysts.12) Other vascular lesions were also excluded from the diagnosis by negative findings on angiography.
The first repeat brain MRI performed 5 months after the initial evaluations showed the typical features of CMs. An enlarging lesion with multi-stage hemorrhage and a rim of hemosiderin deposit were found, suggesting that recurrent bleeding had occurred within the CM. Two other repeat brain imaging studies including CT and MRI were performed, which showed enlargement of the cystic CM consistent with recurrent bleeding. At least two episodes of recurrent bleeding had probably occurred within the previous two months.
The timing of surgery and repeated brain imaging studies has been debated.8)18)20) However, in general, surgical evacuation is preferred if there is evidence of recurrent bleeding from CMs located in non-eloquent and supratentorial regions.2)14) Poorthuis et al.,14) in a review of 62 previously reported studies to quantify the risks associated with CM treatment by surgical excision and stereotactic radiosurgery, concluded that risks from surgery compared favorably with the risk of recurrent bleeding from CMs. The outcomes of surgical excision had improved over time, and the risks were lower in non-brainstem CMs with recurrent hemorrhage and higher in brainstem CMs. Pandey et al.13) concluded that resection of symptomatic deep CMs in areas such as the thalamus and basal ganglia produced acceptable outcomes. Based on the evidence, symptomatic recurrent bleeding from a CM should be treated surgically, as in our case. In addition, a cystic CM should be considered in differential diagnoses even though the intra-parenchymal cystic lesion shows no evidence of solid components or a rim of hemosiderin. In such cases, follow-up imaging studies should be considered, however it is not clear when these should be performed. As mentioned above, the annualized risk of repeat bleeding was reported as 3.8-29.5%, greater than the bleeding risk for a first event. Those reports14)18) did not recommend the exact timing of follow-up studies, although follow-up imaging studies were performed and analyzed annually in many cases. Further studies are needed to establish recommendations for the indication and timing of follow-up imaging studies.
Cystic CMs can present as a pure cyst with an internal fluid-fluid level. Neurosurgeons should consider the likelihood of CMs in cases of cystic cerebral lesions with repeated hemorrhage. Short-term repeat brain imaging studies may be helpful in confirming the diagnosis and predicting the natural course, including the likelihood of further hemorrhage.
References
1. Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995 7;83(1):56-59;


2. Bertalanffy H, Benes L, Miyazawa T, Alberti O, Siegel AM, Sure U. Cerebral cavernomas in the adult: review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev. 2002 3;25(1-2):1-53; discussion 54-5



3. Flemming KD, Link MJ, Christianson TJ, Brown RD. Prospective hemorrhage risk of intracerebral cavernous malformations. Neurology. 2012 2;78(9):632-636;


4. Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 2011 6;30(6):E24


5. Hatachita S, Miyajima M, Koga N. Cystic cavernous angioma-case report. Neurol Med Chir (Tokyo). 1991 7;31(7):414-416;


6. Kadota O, Sakaki S, Kumon Y, Ohta S, Kohno K. Large cystic cavernous angioma of the cerebellum: case report. Neurol Med Chir (Tokyo). 1994 11;34(11):768-772;


7. Kim IC, Kwon KY, Rhee JJ, Lee JW, Hur JW, Lee HK. Giant cystic cerebral cavernous malformation with multiple calcification: case report. J Cerebrovasc Endovasc Neurosurg. 2013 9;15(3):255-259;



8. Kondziolka D, Lunsford LD, Kestle JR. The natural history of cerebral cavernous malformations. J Neurosurg. 1995 11;83(5):820-824;


9. Lehnhardt FG, vonSmekal U, R├╝ckriem B, Stenzel W, Neveling M, Heiss WD, et al. Value of gradient-echo magnetic resonance imaging in the diagnosis of familial cerebral cavernous malformation. Arch Neurol. 2005 4;62(4):653-658;


10. Mathiesen T, Edner G, Kihlstr├Čm L. Deep and brainstem cavernomas: a consecutive 8-year series. J Neurosurg. 2003 7;99(1):31-37;


11. Ohba S, Shimizu K, Shibao S, Nakagawa T, Murakami H. Cystic cavernous angiomas. Neurosurg Rev. 2010 10;33(4):395-400;



12. Osborn AG, Preece MT. Intracranial cysts: radiologicpathologic correlation and imaging approach. Radiology. 2006 6;239(3):650-664;


13. Pandey P, Westbroek EM, Gooderham PA, Steinberg GK. Cavernous malformation of brainstem, thalamus and basal ganglia: a series of 176 patients. Clinical studies. Neurosurgery. 2013 4;72(4):573-589; discussion 588-9



14. Poorthuis MH, Klijn CJ, Algra A, Rinkel GJ, Al-Shahi Salman R. Treatment of cerebral cavernous malformations: a systematic review and meta-regression analysis. J Neurol Neurosurg Psychiatry. 2014 12;85(12):1319-1323;


15. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral cavernous malformations: natural history and prognosis after clinical deterioration with or without hemorrhage. J Neurosurg. 1997 8;87(2):190-197;


16. Ramina R, Ingunza W, Vonofakos D. Cystic cerebral cavernous angioma with dense calcification: case report. J Neurosurg. 1980 2;52(2):259-262;


17. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg. 1991 11;75(5):709-714;


18. Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, Bhattacharya JJ, et al. Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol. 2012 3;11(3):217-224;


19. Samii M, Eghbal R, Carvalho GA, Matthies C. Surgical management of brainstem cavernomas. J Neurosurg. 2001 11;95(5):825-832;


20. Sandalcioglu I, Wiedemayer H, Secer S, Asgari S, Stolke D. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry. 2002 3;72(3):351-355;



21. Sato K, Kubota T. Large calcified cystic cavernous angioma in the thalamus. Neurol Med Chir (Tokyo). 1995 2;35(2):100-103;


22. Siddiqui AA, Jooma R. Neoplastic growth of cerebral cavernous malformation presenting with impending cerebral herniation: a case report and review of the literature on de novo growth of cavernomas. Surg Neurol. 2001 7;56(1):42-45;


Fig.┬Ā1
Initial brain imaging findings for this patient. Axial T1-weighted (A), T2-weighted (B) magnetic resonance images, and computed tomography (C) showed a single cystic mass measuring 16 mm in diameter with a fluid-fluid level in the left basal ganglia. Note that there was no evidence of solid components or a hemosiderin rim. An angiogram (D) showed neither abnormal tumor staining nor vascular malformation.
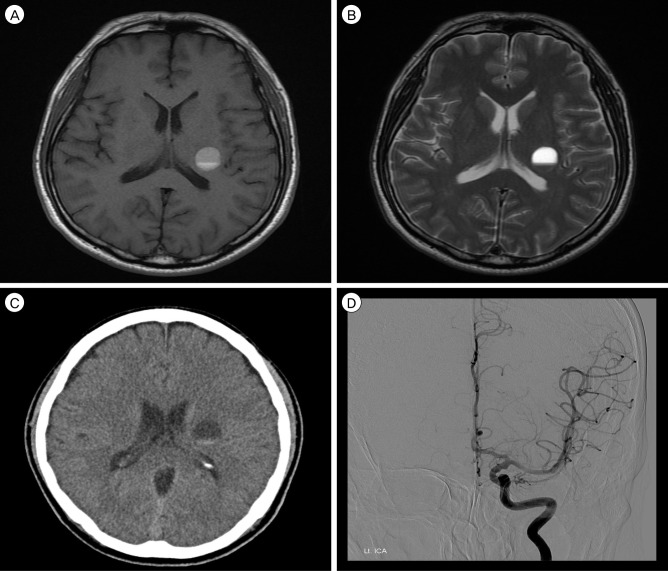
Fig.┬Ā2
First repeated brain imaging performed 5 months after the initial studies (A and B) and preoperative imaging performed 7 months after the initial studies (C and D). Axial T1-weighted (A), T2-weighted (B) magnetic resonance images showed a reticulated "salt and pepper" pattern in the core and a peripheral halo of low signal intensity. Axial T1-weighted (C), and T2-weighted (D) magnetic resonance images showed an increase in the size of the lesion with aggravated peri-lesional edema. Mixed signal intensity in the core with peripheral low signal intensity gradually becomes more distinct.
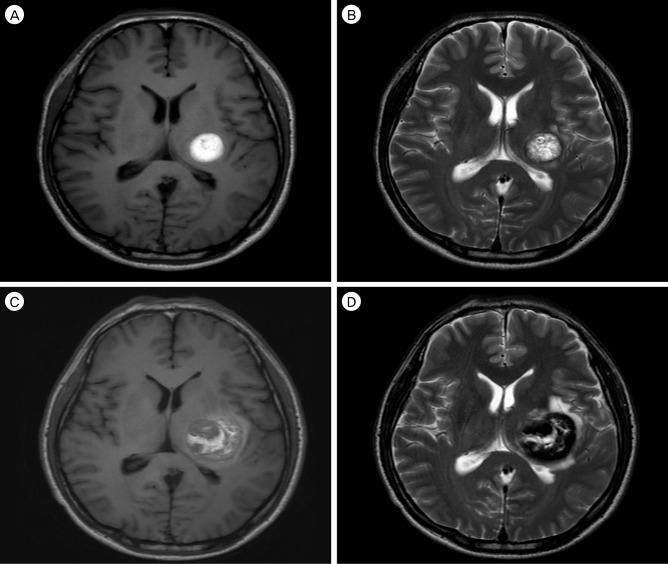
Fig.┬Ā3
In the histopathological examination, the lesion showed irregularly dilated vascular channels lacking a smooth muscle layer. A rim of fibrosis and hemosiderin-laden macrophages on a hemorrhagic background surrounded the lesion. In the lumen of the vascular channels, there were some organizing thrombi. However, there was no evidence of microcalcification. These histopathological findings were consistent with CM. Hematoxylin-eosin staining; magnification ├Ś100 (A and B). A post-operative axial T2-weighted magnetic resonance image (C) shows no residual lesion.
- TOOLS
-
METRICS

-
- 2 Crossref
- 0 Scopus
- 2,260 View
- 24 Download
- Related articles
-
Unusual presentation of basilar artery thrombosis2020 December;22(4)
Endovascular Treatment of Cerebral Aneurysms.1999 March;1(1)
Surgical Management of Large Cerebral Arteriovenous Malformation.1999 March;1(1)
Management of Intracranial Cavernous Malformations.1999 March;1(1)
Endovascular Treatment of Cerebral Vascular Malformations.1999 March;1(1)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



