 |
 |
| J Cerebrovasc Endovasc Neurosurg > Volume 19(4); 2017 > Article |
|
Abstract
Objective
Tentorial dural arteriovenous fistulas usually drain into cortical veins and often present with hemorrhage. Treatment goal is occlusion of the draining vein, either by surgery or endovascular techniques. We present the multimodality treatment results of 12 patients with tentorial dural arteriovenous fistulas.
Materials and Methods
Between January 2007 and January 2017, 12 consecutive patients with tentorial dural arteriovenous fistulas were treated. There were 11 men and 1 woman with a mean age of 62 years (range 44-85). Clinical presentation was hemorrhage in 8 (67%), pulsatile tinnitus in 2 (17%) and an incidental finding in 2 (17%). The fistula location was at the tentorium cerebelli in 5 (42%), the torcula Herophilii in 4 (33%) and petroclival in 3 (25%).
Results
In 11 patients, arterial embolization with Onyx or PHIL was the primary treatment. Complete obliteration was achieved in one session in 5 (45%) and in 2 sessions in 4 (36%). In 2 patients additional surgery was needed. Primary surgery was performed in 1 patient followed by endovascular coil occlusion via the venous route. One patient with exclusive pial feeders from the posterior inferior cerebellar artery had a clinically silent P3 occlusion during trans arterial embolization. Finally, all 12 fistula were completely occluded, confirmed with angiography after 8-12 weeks. There were no permanent procedural complications.
Dural arteriovenous fistulas (DAVFs) are uncommon vascular disorders and account for 10-15% of all intracranial vascular shunts.7)8) They are acquired multiple hole fistulas connecting dural arteries with cortical dural veins or dural sinus. The clinical course of DAVFs is variable depending on the pattern of venous drainage.2) Symptoms range from asymptomatic to an aggressive clinical presentation consisting of hemorrhage, venous hypertension and neurological deficit. Tentorial dural arteriovenous fistulas (TDAVFs) are a rare subgroup and represent up to 4% of DAVFs. They occur in the double dural layer of the tentorium and its attachments.1) TDAVFs commonly drain directly into cortical cerebral or cerebellar veins with inherent high hemorrhage risk.3)8) Therefore complete elimination of the arteriovenous shunt is recommended, irrespective of presentation.9)
DAVFs are characterized as multiple arterial dural feeders draining into a single dural vein. To eliminate the fistula the operator has to disconnect the draining vein from the supplying dural arteries. Incomplete treatment allows recruitment of collateral vessels and persistent risk of hemorrhage. Treatment of DAVFs is traditionally by surgical disconnection of the draining vein but access in tentorial DAVFs may be difficult due to the deep location.4) In recent years endovascular embolization has become a first-line treatment, either alone or in combination with surgery. The optimal method for endovascular treatment remains controversial. A consideration of the advantages and disadvantages of transarterial, transvenous, and combined approaches should be given in each case before proceeding with embolization.6) Another treatment alternative is Gamma Knife radiosurgery, which has been reported in feasibility studies.5)
In this study we evaluate the incidence, clinical presentation, vascular anatomy and multidisciplinary treatment of a single center cohort of 12 TDAVFs.
Between January 2007 and January 2017, 135 patients with dural arteriovenous fistulas were treated in our institution. Fifty patients (37%) had a spinal DAVF. Of 85 cerebral DAVFs, 11 (8%) were located in the cavernous sinus, 61 (45%) drained supratentorally and 12 (9%) were located in the tentorium. These 12 consecutive patients with TDAVF are the subjects of this study. There were 11 men and 1 woman with a mean age of 62 years (median, 63; range, 44-85 years). Clinical presentation was hemorrhage in 8 (67%), pulsatile bruit in 1 (8%) and 2 were an incidental finding (16%). One patient (8%) with a previously endovascular treated tentorial DAVF presented 9 years later with recurrent pulsatile bruit. The primary fistula location was at the tentorium cerebelli in 5 (42%), on the torcula in 4 (33%) and the petroclival tentorial ridge in 3 (25%). Treatment strategy was decided in a multidisciplinary meeting with neuroradiologists, neurosurgeons and neurologists based on imaging and complete cerebral angiography. Endovascular trans arterial approach was the primary treatment of choice.
Endovascular treatment was performed with the patient under general anesthesia on a biplane angiographic unit (Integris Allura Neuro, Philips Healthcare, Best, the Netherlands). A 5 F-guiding catheter was positioned in the internal carotid, external carotid or vertebral artery, and an Onyx-compatible microcatheter (Rebar 105-5081-153, Medtronic, Minneapolis, MN or Scepter, MicroVention, Tustin, CA, USA) was coaxially advanced in a feeding artery as close as possible to the fistula site. Whenever possible, the middle meningeal artery was used for access to the fistula, since this dural artery usually has a straight course allowing for easy distal access. Onyx 18 (Medtronic) or PHIL (MicroVention) was slowly injected. When reflux occurred, the injection was paused for a few minutes. In most cases reflux occurred several times before penetration of Onyx through the fistula site into the draining vein. Once Onyx advanced, it was intermittently injected until the draining veins were filled and all feeders were filled retrograde, thereby completely occluding the fistula. In the last years, the Scepter micro catheter was used. This catheter has a micro balloon just proximal to the tip. When inflated, the balloon prevents reflux in the feeding artery. Immediate angiographic cure was defined as the formation of the cast of the embolic agent in the target fistula as well as the proximal draining veins and no residual arteriovenous shunting. Post procedural pain was managed with medication according to local protocol, in some cases including intravenous morphine or fentanyl. Follow-up angiography was performed after 6-12 weeks. Illustrative cases are provided in Fig. 1, 2, 3.
In 11 of 12 patients, arterial embolization with Onyx or PHIL was the primary treatment. Complete obliteration was achieved in one session in 5 (45%) and in 2 sessions in 4 (36%). In 2 patients additional surgery was needed to completely close the fistula. Surgery was considered first treatment in 1 patient. This patient presented with a large intracranial hemorrhage from a small TDAVF. Surgery was not successful due to the deep location and small fistula size. The fistula was subsequently treated endovascular by coil occlusion of the draining vein via a trans venous approach. One patient who presented in 1994 with unbearable pulsatile bruit was treated with particles (250-350 ┬Ąm) and coils at that time. The fistula persisted, but the bruit decreased and was clinically acceptable. Twenty-three years later, in 2007 the patient returned with agonizing bruit and was then embolized with Onyx achieving complete obliteration. Finally, all 12 fistulae were completely occluded, confirmed with angiography after 8-12 weeks.
One patient with exclusive pial feeders from the posterior inferior cerebellar artery (PICA) had a clinically silent P3 occlusion during trans arterial embolization. There were no permanent adverse events, neither from surgery nor from endovascular treatment (0%, 97.5% CI 0-30%).
Primary endovascular access was the occipital artery in 4 (36%), middle meningeal artery in 3 (27%), posterior meningeal artery in 1 (9%), inferolateral trunk branch in 1 (9%), ascending pharyngeal artery in 1 (9%), and PICA in 1 (9%).
Ten (83%) patients experienced post procedural pain after embolization, hypothetically because of dural ischemia. All patients were managed according to local analgesic protocol. At the time of follow-up angiography 8-12 weeks later, all patients were pain-free without medication. Post procedural radiation induced alopecia did not occur.
TDAVFs are rare acquired lesions comprising a small minority of all treated DAVFs in our institution. Most were symptomatic presenting with hemorrhage or intolerable bruit and the remaining were incidentally discovered on magnetic resonance imaging. In our cohort there was a striking preponderance for males.
All our TDAVFs drained in the cortical venous system. Either the deep venous system, cerebellar veins or the temporo-occipital cortical veins. All but one of the hemorrhagic patients presented with hemorrhage in the posterior fossa. Because of the aggressive natural history and the presence of retrograde venous flow (type III or IV according to Cognard) the treatment goal was complete obliteration of the fistula, even in the patients who presented with pulsatile bruit or with a TDAVF as incidental finding.
TDAVFs are more challenging to treat compared with DAVFs at other locations and may require multiple endovascular or surgical treatment sessions. Surgically these fistulas are difficult to access because of the deep location. Endovascularly they are challenging because there is usually dural supply from both internal carotid artery (ICA) and external carotid artery. Arterial embolization through dural arteries from the ICA increases the procedural risk of intracranial embolic events. There is a vast anastomotic network between the intracranial and extracranial dural circulation around the skull base and reflux of liquid embolic may cause distal intracranial embolic complications. It is well established that the middle meningeal artery is a favorable access to the fistula point in DAVFs. However, in TDAVFs this artery is mostly not involved. In some cases the occipital artery is the only extracranial endovascular access. This artery is surrounded by loose connective tissue and may be very tortuous, especially when it accommodates high flow. The tortuous course makes advancement of the microcatheter difficult. If obliteration of the fistula through the occipital artery is unsuccessful or there is no extracranial dural arterial supply at all, access via dural arteries from the ICA or pio-dural connections via the vertebrobasilar system is the remaining transarterial option. When reflux occurs during embolization of the tentorial artery or posterior meningeal artery, the risk of severe neurological complications is high.
During embolization with Onyx sometimes many dural arterial feeders filled in a retrograde fashion before penetration into the draining vein was achieved. Venous penetration and occlusion is crucial to permanently obliterate the whole fistulous complex.
Most patients reported post procedural pain and some needed pain medication during one or several days. This pain is probably related to relative ischemia of the dura as a result of occlusion of dural arteries. Pain usually gradually decreased in the days following the procedure, and at the time of follow-up angiography after 6-12 weeks all patients were pain free.
In conclusion, the strategy of surgery and endovascular treatment in a multidisciplinary management of patients with TDAVFs led to stable angiographic cure in all patients without permanent procedural complications. TDAVFs are challenging to treat because of deep location and frequent dural feeders from internal carotid and vertebral artery. Multiple sessions were necessary to completely obliterate the fistulas in the majority of patients.
References
1. Byrne JV, Garcia M. Tentorial dural fistulas: endovascular management and description of the medial dural-tentorial branch of the superior cerebellar artery. AJNR Am J Neuroradiol. 2013 9;34(9):1798-1804;



2. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995 3;194:671-680;


3. Daniels DJ, Vellimana AK, Zipfel GJ, Lanzino G. Intracranial hemorrhage from dural arteriovenous fistulas: clinical features and outcome. Neurosurg Focus. 2013 5;34(5):E15.


4. Davies MA, Ter Brugge K, Willinsky R, Wallace MC. The natural history and management of intracranial dural arteriovenous fistulae. Part 2: aggressive lesions. Interv Neuroradiol. 1997 12;3(4):303-311;


5. Dmytriw AA, Schwartz ML, Cusimano MD, Mendes Pereira V, Krings T, Tymianski M, et al. Gamma Knife radiosurgery for the treatment of intracranial dural arteriovenous fistulas. Interv Neuroradiol. 2017 4;23:211-220;


6. Gandhi D, Chen J, Pearl M, Huang J, Gemmete JJ, Kathuria S. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol. 2012 6;33:1007-1013;



7. Hiramatsu M, Sugiu K, Hishikawa T, Haruma J, Tokunaga K, Date I, et al. Epidemiology of dural arteriovenous fistula in Japan: Analysis of Japanese Registry of Neuroendovascular Therapy (JR-NET2). Neurol Med Chir (Tokyo). 2014 1;54(1):63-71.

Fig.┬Ā1
A 50-year-old male with a right occipital hematoma. (A) Computed tomography scan showing the occipital hematoma. (B) Left vertebral artery angiogram demonstrates a small tentorial dural fistula supplied by the medial dural branch of the superior cerebellar to a deep cortical vein and ultimately draining into the straight sinus. Arrow shows drainage point in the straight sinus. (C) Trans venous navigation via the straight sinus into the draining cortical vein. (D) Lateral radiograph showing the final coil mass in the cortical draining vein close to the straight sinus. (E) Left vertebral angiogram demonstrates obliteration of the fistula with patent straight sinus.
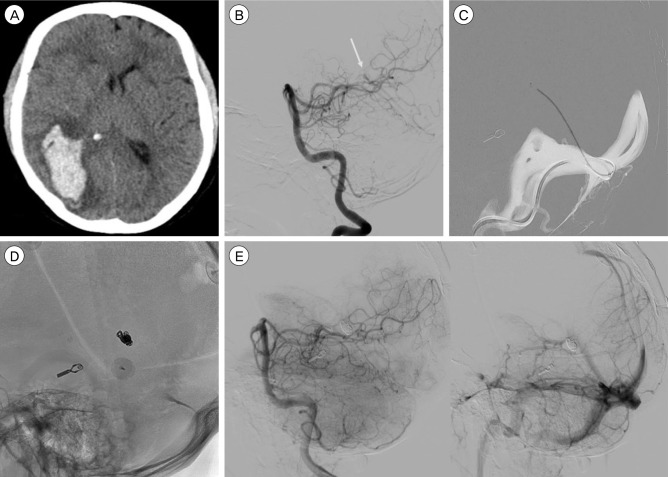
Fig.┬Ā2
A 57-year-old male presenting with debilitating tinnitus. (A) Lateral angiogram demonstrating a petroclival tentorial dural arteriovenous fistula with feeders from the inferolateral trunk and meningohypophyseal trunk. (B) Lateral angriogram with external carotid artery injection demonstrating feeders from the middle meningeal artery (MMA) and occipital artery. (C) Digital subtraction image demonstrating distal catheter access via the MMA. (D) Final Onyx (Medtronic) cast demonstrating obliteration of the fistula.
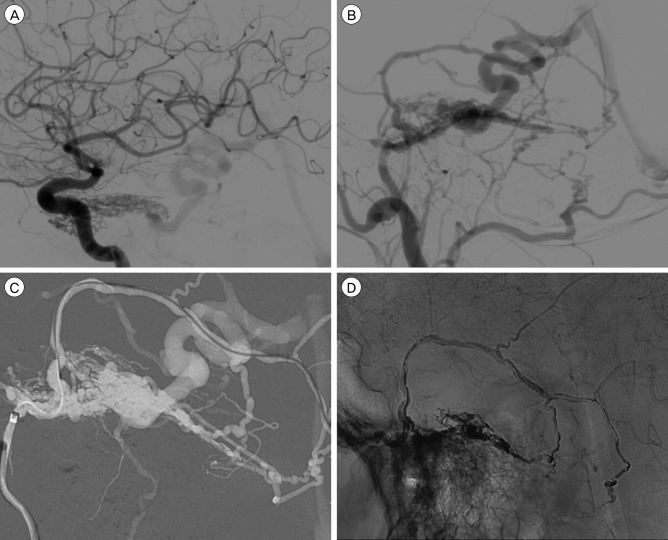
Fig.┬Ā3
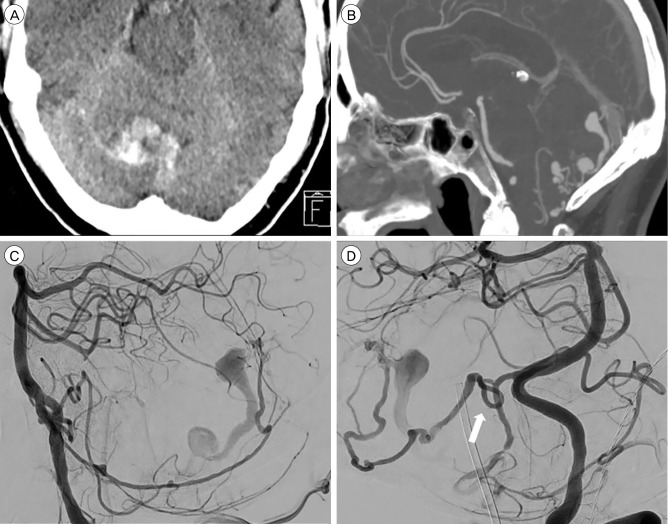
A 63-year-old male presenting with right cerebellar hemorrhage. (A) Axial computed tomography (CT) scan demonstrating a right cerebellar hemorrhage. (B) CT angiography sagittal reconstruction demonstrating venous ectasia and fistulapoint at the torcula herophilii. (C) Left vertebral injection. Lateral angiogram demonstrating main arterial feeder from the posterior meningeal artery (PM). (D) Left vertebral injection. Arrow demonstrating PM loop where the PM is crossing the tentorial ridge. Distal endovascular access proved impossible because of this loop. Because no other viable arteries were available, the patient went for surgery.
Table┬Ā1
Patient characteristics
- TOOLS
-
METRICS

-
- 9 Crossref
- 0 Scopus
- 2,940 View
- 45 Download
- Related articles
-
Treatment of Superior Sagittal Sinus Dural Arteriovenous Fistula.2007 June;9(2)
Intracranial Pial Arteriovenous Fistula Presenting with Hemorrhage: A Case Report2012 December;14(4)




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Full text via PMC
Full text via PMC Download Citation
Download Citation Print
Print



